Abstract
Purpose
The aim of the current study was to investigate some of the key regulators of mitochondrial oxidative metabolism in ESRD patients on hemodialysis (ESRD/HD) focusing on peroxisome proliferator-activated receptor-γ–coactivator 1α (PGC-1α) gene expression and its relation to ESRD/HD-related cardiovascular diseases (CVD) and mortality in an effort to identify new potential targets for pharmacological interventions.
Subjects and methods
The expression of PGC-1α and one of its downstream genes: COX6C were evaluated in 49 ESRD/HD patients and in 33 age- and sex-matched healthy subjects as controls using quantitative real-time PCR. Malondialdehyde (MDA) was measured using colorimetric method as a marker of oxidative stress. Patients were followed up for 24 months for the development of HD-related cardiovascular complications and mortality.
Results
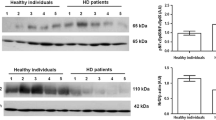
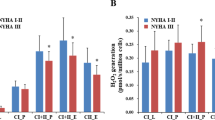
PGC-1α and COX6C expressions were significantly down-regulated in ESRD/HD patients compared to the controls (P ≤ 0.001 for both). Additionally, MDA level was higher in HD patients (P ≤ 0.001). Negative correlation was found between PGC-1α expression and MDA level (P ≤ 0.001). MDA was significantly higher, while PGC-1α expression was significantly lower in HD patients who developed CVD than in patients who did not. By using multivariate logistic regression analysis, it was found that down-regulated PGC-1α expression is independently associated with the development of CVD in HD patients.
Conclusion
Our study suggests that ESRD/HD patients might have oxidative mitochondrial dysfunction, which may be partially responsible for CKD-related cardiovascular complications. Pharmacological modulation of PGC-1α might be a promising therapeutic tool to reduce oxidative stress-related complications in ESRD/HD patients.






Similar content being viewed by others
References
Al-Nimer MS, Jaleel NA (2012) Assessment of nitrogen radicals and their scavenging activity in patients with end-stage renal failure. Saudi J Kidney Dis Transpl 23:290–295
Oberg BP, McMenamin E, Lucas FL, McMonagle E, Morrow J, Ikizler TA et al (2004) Increased prevalence of oxidant stress and inflammation in patients with moderate to severe chronic kidney disease. Kidney Int 65:1009–1016
Hansson GK (2005) Inflammation, atherosclerosis, and coronary artery disease. N Engl J Med 352:1685–1695
Locatelli F, Canaud B, Eckardt KU, Stenvinkel P, Wanner C, Zoccali C (2003) Oxidative stress in end-stage renal disease: an emerging threat to patient outcome. Nephrol Dial Transpl 18:1272–1280
de Jager DJ, Grootendorst DC, Jager KJ, van Dijk PC, Tomas LM, Ansell D et al (2009) Cardiovascular and noncardiovascular mortality among patients starting dialysis. JAMA 302:1782–1789
Cibulka R, Racek J (2007) Metabolic disorders in patients with chronic kidney failure. Physiol Res 56:697–705
Canaud B, Cristol J, Morena M, Leray-Moragues H, Bosc J, Vaussenat F (1999) Imbalance of oxidants and antioxidants in hemodialysis patients. Blood Purif 17:99–106
Zaza G, Pontrelli P, Pertosa G, Granata S, Rossini M, Porreca S et al (2008) Dialysis-related systemic microinflammation is associated with specific genomic patterns. Nephrol Dial Transpl 23:1673–1681
Finck BN, Kelly DP (2006) PGC-1 coactivators: inducible regulators of energy metabolism in health and disease. J Clin Invest 116:615–622
Pedraza-Chaverri J, Sánchez-Lozada LG, Osorio-Alonso H, Tapia E, Scholze A (2016) New pathogenic concepts and therapeutic approaches to oxidative stress in chronic kidney disease. Oxid Med Cell Longev 2016:6043601
Liang H, Ward WF (2006) PGC-1alpha: a key regulator of energy metabolism. Adv Physiol Educ 30:145–151
Finkel T (2006) Cell biology: a clean energy programme. Nature 444:151–152
Zaza G, Granata S, Masola V, Rugiu C, Fantin F, Gesualdo L et al (2013) Downregulation of nuclear-encoded genes of oxidative metabolism in dialyzed chronic kidney disease patients. PLoS ONE 8:e77847. doi:10.1371/journal.pone.0077847
Herbelin A, Urena P, Nguyen AT, Zingraff J, Descamps-Latscha B (1991) Elevated circulating levels of interleukin-6 in patients with chronic renal failure. Kidney Int 39:954–960
Granata S, Zaza G, Simone S, Villani G, Latorre D, Pontrelli P et al (2009) Mitochondrial dysregulation and oxidative stress in patients with chronic kidney disease. BMC Genom 10:388. doi:10.1186/1471-2164-10-388
Akahoshi T, Kobayashi N, Hosaka S, Sekiyama N, Wada C, Kondo H (1995) In-vivo induction of monocyte chemotactic and activating factor in patients with chronic renal failure. Nephrol Dial Transpl 10:2244–2249
Lonnemann G, van der Meer JW, Cannon JG, Dinarello CA, Koch KM, Granolleras C et al (1987) Induction of tumor necrosis factor during extracorporeal blood purification. N Engl J Med 317:963–964
Becker BN, Himmelfarb J, Henrich WL, Hakim RM (1997) Reassessing the cardiac risk profile in chronic hemodialysis patients: a hypothesis on the role of oxidant stress and other non-traditional cardiac risk factors. J Am Soc Nephrol 8:475–486
Serviddio G, Romano AD, Cassano T, Bellanti F, Altomare E, Vendemiale G (2011) Principles and therapeutic relevance for targeting mitochondria in aging and neurodegenerative diseases. Curr Pharm Des 17:2036–2055
Rains JL, Jain SK (2011) Oxidative stress, insulin signaling, and diabetes. Free Radic Biol Med 50:567–575
Chatterjee A, Dasgupta S, Sidransky D (2011) Mitochondrial subversion in cancer. Cancer Prev Res (Phila) 4:638–654
Widlansky ME, Gutterman DD (2011) Regulation of endothelial function by mitochondrial reactive oxygen species. Antioxid Redox Signal 15:1517–1530
West AP, Shadel GS, Ghosh S (2011) Mitochondria in innate immune responses. Nat Rev Immunol 11:389–402
Mitchell P (1976) Possible molecular mechanisms of the protonmotive function of cytochrome systems. J Theor Biol 62:327–367
Yagi K (1998) Simple assay for the level of total lipid peroxides in serum or plasma. Methods Mol Biol 108:101–106
Breborowicz A (1992) Free radicals in peritoneal dialysis: Agents of damage? Perit Dial Int 12:194–198
Tarng DC, Wen Chen T, Huang TP, Chen CL, Liu TY, Wei YH (2002) Increased oxidative damage to peripheral blood leukocyte DNA in chronic peritoneal dialysis patients. J Am Soc Nephrol 13:1321–1330
Raj DS, Boivin MA, Dominic EA, Boyd A, Roy PK, Rihani T et al (2007) Hemodialysis induces mitochondrial dysfunction and apoptosis. Eur J Clin Invest 37:971–977
Rice JB, Stoll LL, Li WG, Denning GM, Weydert J, Charipar E et al (2003) Low-level endotoxin induces potent inflammatory activation of human blood vessels: inhibition by statins. Arterioscler Thromb Vasc Biol 23:1576–1582
Borniquel S, García-Quintáns N, Valle I, Olmos Y, Wild B, Martínez-Granero F et al (2010) Inactivation of Foxo3a and subsequent downregulation of PGC-1α mediate nitric oxide-induced endothelial cell migration. Mol Cell Biol 30:4035–4044
Dugan LL, You YH, Ali SS, Diamond-Stanic M, Miyamoto S, DeCleves AE et al (2013) AMPK dysregulation promotes diabetes-related reduction of superoxide and mitochondrial function. J Clin Invest 123:4888–4899
Rasbach KA, Schnellmann RG (2007) PGC-1alpha over-expression promotes recovery from mitochondrial dysfunction and cell injury. Biochem Biophys Res Commun 355:734–739
Gamboa JL, Billings FTT, Bojanowski MT, Gilliam LA, Yu C, Roshanravan B et al (2016) Mitochondrial dysfunction and oxidative stress in patients with chronic kidney disease. Physiol Rep 4:e12780. doi:10.14814/phy2.12780
Adey D, Kumar R, McCarthy JT, Nair KS (2000) Reduced synthesis of muscle proteins in chronic renal failure. Am J Physiol Endocrinol Metab 278:E219–E225
Lewis MI, Fournier M, Wang H, Storer TW, Casaburi R, Cohen AH et al (2012) Metabolic and morphometric profile of muscle fibers in chronic hemodialysis patients. J Appl Physiol 112:72–78
Rao M, Li L, Demello C, Guo D, Jaber BL, Pereira BJ et al (2009) Mitochondrial DNA injury and mortality in hemodialysis patients. J Am Soc Nephrol 20:189–196
Guo K, Lu J, Huang Y, Wu M, Zhang L, Yu H et al (2015) Protective role of PGC-1alpha in diabetic nephropathy is associated with the inhibition of ROS through mitochondrial dynamic remodeling. PLoS ONE 10:e0125176. doi:10.1371/journal.pone.0125176
Baldelli S, Aquilano K, Ciriolo MR (2014) PGC-1alpha buffers ROS-mediated removal of mitochondria during myogenesis. Cell Death Dis 5:e1515
Hambali Z, Ahmed Z, Arab S, Khazaai H (2011) Oxidative stress and its association with cardiovascular disease in chronic renal failure patients. Indian J Nephrol 21:21–25
Rowe GC, Jiang A, Arany Z (2010) PGC-1 coactivators in cardiac development and disease. Circ Res 107:825–838
Sano M, Wang SC, Shirai M, Scaglia F, Xie M, Sakai S et al (2004) Activation of cardiac Cdk9 represses PGC-1 and confers a predisposition to heart failure. EMBO J 23:3559–3569
Sun CK, Chang LT, Sheu JJ, Wang CY, Youssef AA, Wu CJ et al (2007) Losartan preserves integrity of cardiac gap junctions and PGC-1α gene expression and prevents cellular apoptosis in remote area of left ventricular myocardium following acute myocardial infarction. Int Heart J 48:533–545
Honda T, Kaikita K, Tsujita K, Hayasaki T, Matsukawa M, Fuchigami S et al (2008) Pioglitazone, a peroxisome proliferators-activated receptor-gamma agonist, attenuates myocardial ischemia-reperfusion injury in mice with metabolic disorders. J Moll Cell Cardiol 44:915–926
Fabregat-Andres O, Paredes F, Monsalve M, Milara J, Ridocci-Soriano F, Gonzalez-Hervas S et al (2016) mRNA PGC-1α levels in blood samples reliably correlates with its myocardial expression: study in patients undergoing cardiac surgery. Anatol J Cardiol 16:622–629
Hofer A, Noe N, Tischner C, Kladt N, Lellek V, Schauss A et al (2014) Defining the action spectrum of potential PGC-1alpha activators on a mitochondrial and cellular level in vivo. Hum Mol Genet 23:2400–2415
Bastin J, Aubey F, Rotig A, Munnich A, Djouadi F (2008) Activation of peroxisome proliferator-activated receptor pathway stimulates the mitochondrial respiratory chain and can correct deficiencies in patients’ cells lacking its components. J Clin Endocrinol Metab 93:1433–1441
Lagouge M, Argmann C, Gerhart-Hines Z, Meziane H, Lerin C, Daussin F et al (2006) Resveratrol improves mitochondrial function and protects against metabolic disease by activating SIRT1 and PGC-1alpha. Cell 127:1109–1122
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
The authors declare that they have no conflicts of interest that are directly relevant to the content of this article.
Ethical standards
This research was conducted on human participants. The study was approved by the Ethics Committee of Alexandria University, Egypt.
Informed consent
Informed consent was obtained from all individual participants included in the study.”
Rights and permissions
About this article
Cite this article
Elsayed, E.T., Nassra, R.A. & Naga, Y.S. Peroxisome proliferator-activated receptor-γ–coactivator 1α (PGC-1α) gene expression in chronic kidney disease patients on hemodialysis: relation to hemodialysis-related cardiovascular morbidity and mortality. Int Urol Nephrol 49, 1835–1844 (2017). https://doi.org/10.1007/s11255-017-1628-5
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11255-017-1628-5