Abstract
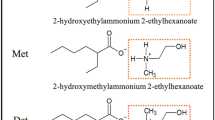
There is a growing interest in the use of ionic liquids to provide lubrication for challenging contacts. This study is an initial assessment of the application of two ionic liquids based on choline chloride cations to be used as ionic liquid lubricants for engineering contacts, in this case steel on steel. These ionic liquids, termed ethaline and reline, have anions of ethylene glycol and urea, respectively, and are available at relatively low costs and in high quantities. In order to assess the lubrication performance of the ionic liquids, lubricated reciprocating sliding wear tests were conducted between M2 tool steel samples and a steel stylus. Initial tests conducted at a sliding speed of 0.005 m s−1 and 30 N showed that ionic liquids could provide low friction lubrication, comparable to that of SAE 5W30 friction modifier free engine oil under the same test conditions; however, lubrication was lost after short sliding distances. Further testing with higher sliding speed/lower load and varying sample surface textures showed that ionic liquid lubrication could be better maintained in high-speed/low-load testing and by increasing the roughness and therefore surface area of the sample. It was also observed that the choline chloride/urea ionic liquid formed a residual film when tested on iron silicate peened samples, and that this film may promote lubrication.







Similar content being viewed by others

References
Welton, T.: Room-temperature ionic liquids—solvents for synthesis and catalysis. Chem. Rev. 99(8), 2071 (1999)
Earle, M.J., Seddon, K.R.: Ionic liquids—green solvents for the future. Pure Appl. Chem. 72(7), 1391–1398 (2000)
Ohno, H.: Electrochemical Aspects of Ionic Liquids. Wiley-Interscience, New York (2005)
Wasserscheid, P., Keim, W.: Ionic liquids—new “solutions” for transition metal catalysis. Angew. Chem. Int. Ed. 39, 3772 (2000)
Abbott, A.P., Capper, G., Shikotra, P.: Processing metal oxides using ionic liquids. Mineral Process. Extract. Metall. 115(1), 15–18 (2006)
Abbott, A.P., Bell, T., Handa, S., Stoddart, B.: Cationic functionalisation of cellulose using ionic liquids. Green Chem. 8, 784–786 (2006)
Abbott, A.P., McKenzie, K.J.: Electrodeposition of metals using ionic liquids. Phys. Chem. Chem. Phys. 8(37), 4265–4279 (2006)
Abbott, A.P., Capper, G., McKenzie, K.J., Obi, S.U.: Solubility of metal oxides in deep eutectic solvents based on choline chloride. J. Chem. Eng. Data 51, 1280–1282 (2006)
Abbott, A.P., Capper, G., McKenzie, K.J., Ryder, K.S.: Electrodeposition of zinc tin alloys from deep eutectic solvents based on choline chloride. J. Electroanal. Chem. 599, 288 (2007)
Abbott, A.P., Nandhra, S., Ryder, K.S., Smith, E.: Electroless deposition of metallic silver from a choline chloride based ionic liquid: a study using acoustic impedance spectroscopy, SEM and atomic force microscopy. Phys. Chem. Chem. Phys. 9, 3735–3743 (2007)
Bhatt, A.I., Bond, A.M., Zhang, J.: Electrodeposition of lead on glassy carbon and mercury film electrodes from a distillable room temperature ionic liquid—DIMCARB. J. Solid State Electrochem. 11, 1593 (2007)
Ye, C., Liu, W., Chen, Y., Yu, L.: Room-temperature ionic liquids: a novel versatile lubricant. Chem. Commun. 21, 2244–2245 (2001)
Reich, R.A., Stewart, P.A., Bohaychick, J., Urbanski, J.A.: Base oil properties of ionic liquids. Lubr. Eng. 7 16–21 (2003)
Wang, H.Z., Lu, Q.M., Ye, C.F., Liu, W.M., Cui, Z.J.: Friction and wear behaviours of ionic liquid of alkylimidazolium hexafluorophosphates as lubricants for steel/steel contact. Wear 256, 44–48 (2004)
Liu, W., Ye, C., Gong, Q., Wang, H., Wang, P.: Tribological performance of room-temperature ionic liquids as lubricant. Tribol. Lett. 13, 81–85 (2002)
Yu, G., Zhou, F., Liu, W., Liang, Y., Yan, S.: Preparation of functional ionic liquids and tribological investigation of their ultra-thin films. Wear 260(9–10), 1076–1080 (2006)
Abbott, A.P., Macfarlane, D.R.: In: Endres, F. (ed.) Electrodeposition from ionic liquids, Wiley-VCH, Weinheim (2008). ISBN: 978-3-527-31565-9.
Abbott, A.P., Barron, J.C., Elhadi, M., Frisch, G., Gurman, S.J., Hillman, A.R., Smith, E.L., Mohamoud, M.A., Ryder, K.S.: Electrolytic metal coatings and metal finishing using ionic liquids. ECS Trans. 16(36), 47 (2009)
Abbott, A.P., Harris, R.C., Ryder, K.S.: Application of hole theory to define ionic liquids by their transport properties. J. Phys. Chem. B 111, 4910 (2007)
Abbott, A.P., Capper, G., Davies, D.L., Rasheed, R.K., Tambyrajah, V.: Novel solvent properties of choline chloride/urea mixture. Chem. Commun. 1, 70–71 (2003)
Abbott, A.P.: Application of hole theory to the viscosity of ionic and molecular liquids. ChemPhysChem 5, 1242 (2004)
Acknowledgements
S.D.A. Lawes would like to thank the EPSRC and Jaguar Cars Ltd. for their financial support of this research. The authors would like to acknowledge the contribution of Mr. G. Kolomyjec. Mr. T. Forryan and Mr. G. Clark are thanked for their technical assistance. The authors would also like to thank the EU for funding project IONMET (under FP6, www.ionmet.eu).
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Lawes, S.D.A., Hainsworth, S.V., Blake, P. et al. Lubrication of Steel/Steel Contacts by Choline Chloride Ionic Liquids. Tribol Lett 37, 103–110 (2010). https://doi.org/10.1007/s11249-009-9495-6
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11249-009-9495-6