Abstract
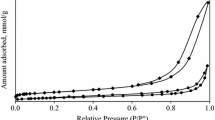
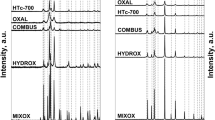
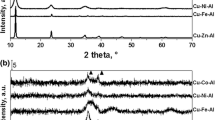
A process of transforming hydrotalcite, containing Cu–Zn–Al (2:1:1) to a catalysts for selective hydrogenolysis of glycerol to 1,2-propanediol has been studied. A two steps process, consisted of the calcination at increased temperature and subsequent reduction with hydrogen, as well as a one step process, in which the hydrotalcite was heated at hydrogen atmosphere so the calcination and reduction were joined to one operation, were verified experimentally. The influence of the temperature in the calcination step in the two steps process on composition, texture and catalytic properties of the active form of catalyst has been studied in detail. X Ray diffraction, thermogravimetric analyses, nitrogen physisorption, mercury porosimetry, temperature-programmed reduction and high-resolution transmission electron microscopy were used for the catalysts characterization. A study of calcination of a Cu–Zn–Al containing hydrotalcite at different temperatures showed, that at temperature ending with 350 °C, predominantly amorphous, mixed oxides containing phase was formed. The most active and most selective catalyst was obtained by the reduction of this amorphous oxide phase. In contrary, highly crystalline material was obtained at higher calcination temperatures, i.e at 450 or 700 °C respectively, but the activity of the reduced form of these crystalline oxides was lower. It was also proven, that by simultaneous calcination and reduction, a catalyst of the same activity and even better selectivity towards 1,2-propanediol could be prepared, comparing with the catalyst prepared by the two steps method.











Similar content being viewed by others
References
Zhou C, Zhao H, Tong D, Wu L, Yu W (2013) Catal Rev: Sci Eng 55:369–453. https://doi.org/10.1080/01614940.2013.816610
Ruppert AM, Weinberg K, Palkovits R (2012) Angewandte Chem Int Ed 51:2564–2601. https://doi.org/10.1002/anie.201105125
Jean D,. Nohair B, Bergeron J, Kaliaguine S (2014) Ind Eng Chem Res 53:18740–18749.
Du Y, Wang C, Jiang H, Chen C, Chen R (2016) J Ind Eng Chem 35:262–267. https://doi.org/10.1016/j.jiec.2016.01.002
Balaraju M, Jagadeeswaraiah K, Sai Prasad BS, Lingaiah N (2012) Catal Sci Technol 2:1967–1976
Xiao Z, Li C, Xiu J, Wang X, Williams CT, Liang C (2012) J Mol Catal A 365:24–31. https://doi.org/10.1016/j.molcata.2012.08.004
Chiu CW, Dasari MA, Suppes GJ, Sutterlin WR (2006) AICHE J 52:3543–3548. https://doi.org/10.1002/aic
Bienholz A, Schwab F, Claus P (2010) Green Chem 12:290–295. https://doi.org/10.1039/b914523k
Liu Y, Pasupulety N, Gunda K, Rempel G, Flora TT (2014) Top Catal 57:1454–1462. https://doi.org/10.1007/s11244-014-0318-O
Pudi S et al (2014) Int J Chem React Eng 12:1–12. https://doi.org/10.1515/ijcre-2013-0102
Valencia R, Tirado JA, Sotelo R, Trejo F, Lartundo L (2015) React Kinet Mech Catal 116:205–222. https://doi.org/10.1007/s11144-015-0885-5
Meher LC, Gopinath R, Naik SN, Dalai AK (2009) Ind Eng Chem Res 48:1840–1846. https://doi.org/10.1021/ie8011424
Kolena J. Soukupová L, Kocík J, Lederer J (2017) React Kinet Mech Catal. https://doi.org/10.1007/s11144-017-1239-2
Kumar P, Srivastava CV, Moshra IM (2015) Energy Fuels 29:2664–2675. https://doi.org/10.1021/ef502856z
Zhang LH, Zheng C, Li F, Evans DG, Duan X (2008) J Mater Sci 43:237–243. https://doi.org/10.1007/s10853-007-2167-8
Cavani F, Trifiro F, Vaccari A (1991) Catal Today 11:173–301
Fornasari G, Gazzano M, Matteuzzi D, Trifiro F, Vaccari A (1995) Appl Clay Sci 10:1069–1082
Casenave S, Martinez H, Guimon C, Auroux A, Hulea V, Dumitriu E (2003) J Therm Anal Calorim 72:191–198
Xia S, Yuan Z, Wang L, Chen P, Hou Z (2011) Appl Catal A 403:173–182. https://doi.org/10.1016/j.apcata.2011.06.026
Xia S, Zheng L, Nie R, Chen P, Lou H, Hou Z (2013) Chin J Catal 34:986–992. https://doi.org/10.1016/S1872-2067(11)60505-6
Yuan ZL, Wang JH, Wang LN, Xie WH, Chen P, Hou ZY, Zheng XM (2011) Appl Catal B 101:431–440. https://doi.org/10.1016/j.apcatb.2010.10.013
Yan N, Dyson PJ 2013, Curr Opin Chem Eng. https://doi.org/10.1016/j.coche.2012.12.004
Lábár JL (2008) Microsc Microanal 14:287–295. https://doi.org/10.1017/S1431927608080380
Lábár JL (2009) Microsc Microanal 15:120–129. https://doi.org/10.1017/S1431927609090023
Lábár JL (2012) Microsc Microanal 18:406–420. https://doi.org/10.1017/S1431927611012803
JCPDS PDF-2 database (2004) International Centre for Diffraction Data. Newtown Square, PA, Release 54
Acknowledgements
This publication is a result of the project Development of the UniCRE Centre (LO1606) which has been financially supported by the Ministry of Education, Youth and Sports of the Czech Republic under the National Sustainability Programme I. The results were achieved using the infrastructure of the project Efficient Use of Energy Resources Using Catalytic Processes (LM2015039) which has been financially supported by MEYS within the targeted support of large infrastructures.
Author information
Authors and Affiliations
Corresponding author
Electronic supplementary material
Below is the link to the electronic supplementary material.
Rights and permissions
About this article
Cite this article
Kolena, J., Skuhrovcová, L., Kocík, J. et al. Catalyst for Selective Hydrogenolysis of Glycerol, Prepared from Hydrotalcite-Like Structures. Top Catal 61, 1746–1756 (2018). https://doi.org/10.1007/s11244-018-1005-3
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11244-018-1005-3