Abstract
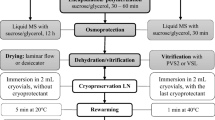
We describe an encapsulation–dehydration procedure with prefreezing steps for the cryopreservation of rhizome bud explants of Asparagus officinalis L. cv. Morado de Huétor. With this procedure, survival of Rhizome buds was at least 84 and 42% developed to complete plantlets at 8 weeks. Flow cytometry and EST-SSR molecular markers were used to assess genetic stability of the regenerated material. Effects of preculture time in a medium rich in sucrose and prefreezing treatments (0 °C or/and − 20 °C) on plant recovery were evaluated. Rhizome Buds of the “Morado de Huétor” landrace were incubated in preculture medium (MS + 0.3 M sucrose) for 48 h, encapsulated in alginate beads and desiccated until a water content of 35%, prefrozen for one hour at 0 °C plus one hour at − 20 °C, followed by cryopreservation in liquid nitrogen, and then were rewarmed and recovered in ARBM medium for 6 weeks and finally incubated in ARBM-0 for 4 weeks. Analyses of ploidy and molecular stability of plantlets recovered from cryopreserved rhizome buds of two selected genotypes showed no differences compared with the mother plants. Cryopreservation of RB explants of A. officinalis with this Encapsulation–Dehydration procedure will be useful in long-term preservation programs.

Similar content being viewed by others
References
Araki H, Shimazaki H, Hirata Y, Oridate T, Harada T, Yakuwa T (1992) Chromosome number variation of callus cells and regenerated plants in Asparagus officinalisL. Plant Tissue Cult Lett 9:169–175
Bouman H, de Klerk GJ (1990) Cryopreservation of lily meristems. Acta Hortic 266:331–337
Carmona-Martín E, Regalado JJ, Padilla IMG, Westendorp N, Encina CL (2014) A new and efficient micropropagation method and its breeding applications in Asparagusgenera. Plant Cell Tissue Organ Cult 119:479–488
Caruso M, Federici CT, Roose ML (2008) EST-SSR markers for asparagus genetic diversity evaluation and cultivar identification. Mol Breed 21:195–204. https://doi.org/10.1007/s11032-007-9120-z
Castillo NRF, Bassil NV, Wada S, Reed BM (2010) Genetic stability of cryopreserved shoot tips of Rubus germplasm. In Vitro Cell Dev Biol Plant 46:246–256. https://doi.org/10.1007/s11627-009-9265-z
Condello E, Palombi MA, Tonelli MG, Damiano C, Caboni E (2009) Genetic stability of wild pear (Pyrus pyrasterBurgsd) after cryopreservation by encapsulation dehydration. Agric Food Sci 18:136–143
Dereuddre J, Scottez C, Arnaud Y, Duron M (1990) Effects of cold hardening on cryopreservation of axillary pear (Pyrus communisL. cv. Beurre Hardy) in vitroplantlets to dehydration and subsequent freezing in liquid nitrogen: effects of previous cold hardening. CR Acad Sci Paris 310, Ser III: 317–323
Engelmann F, Gonzalez-Arnao MT, Wu Y, Escobar R (2008) The development of encapsulation dehydration. In: Reed BM (ed) Plant cryopreservation: a practical guide. Springer, Corvallis, pp 59–75
Fabre J, Dereuddre J (1990) Encapsulation–dehydration: a new approach to cryopreservation of Solanum shoots tips. Cryo Lett 11:413–426
Fernandes P, Rodriguez E, Pinto G, Roldán-Ruiz I, De Loose M, Santos C (2008) Cryopreservation of Quercus subersomatic embryos by encapsulation–dehydration and evaluation of genetic stability. Tree Physiol 28:1841–1850
Galbraight DW, Harkins KR, Maddox JM, Ayres NM, Sharma DP, Firoozabady E (1983) Rapid flow cytometric analysis of the cell cycle intact plant tissues. Science 220:1049–1051
González-Benito ME, Kremer C, Ibáñez MA, Martín C (2016) Effect of antioxidants on the genetic stability of cryopreserved mint shoot tips by encapsulation–dehydration. Plant Cell Tissue Organ Cult 127:359–368
Harding K (1997) Stability of the ribosomal RNA genes in Solanum tuberosum L. plants recovered from cryopreservation. Cryo Lett 18:217–230
Harding K (2004) Genetic integrity of cryopreserved plant cells: a review. Cryo Lett 25:3–22
Jitsuyama Y, Suzuki T, Harada T, Fujikawa S (2002) Sucrose incubation increases freezing tolerance of Asparagus (Asparagus officinalisL.) embryogenic cell suspensions. Cryo Lett 23:103–112
Kohmura H, Sakai A, Chokyu S, Yakuwa T (1992) Cryopreservation of in vitro-cultured multiple bud clusters of asparagus (Asparagus officinalisL. cv. Hiroshimagreen (2n = 30) by the techniques of vitrification. Plant Cell Rep 11:433–437
Kumu Y, Harada T, Yakuwa T (1983) Development of a whole plant from a shoot tip of Asparagus officinalisL. frozen down to –196 °C. J Fac Agric Hokkaido Univ 61(3):285–294
Kunitake H, Mii M (1998) Somatic embryogenesis and its application for breeding and micropropagation in asparagus(Asparagus officinalisL.). Plant Biotechnol 15:51–61
Kunitake H, Nakashima T, Mori K, Tanaka M (1998) Somaclonal and chromosomal effects of genotype, ploidy and culture duration in Asparagus officinalis L. Euphytica 102:309–316
Liu YG, Liu LX, Wang L, Gao AY (2008) Determination of genetic stability in surviving apple shoots following cryopreservation by vitrification. Cryo Lett 29:7–14
Martín C, González-Benito ME (2005) Survival and genetic stability of Dendranthema grandifloraTzevelev shoot apices after cryopreservation by vitrification and encapsulation–dehydration. Cryobiology 51:281–289
Martín C, Cervera MT, González-Benito MT (2011) Genetic stability analysis of chrysanthemum (Chrysanthemum x morifoliumRamat) after different stages of an encapsulation–dehydration cryopreservation protocol. J Plant Physiol 168:158–166
Mix-Wagner G, Conner AJ, Cross RJ (2000) Survival and recovery of asparagus shoot tips after cryopreservation using the droplet method. N Z J Crop Hortic Sci 28:283–287
Moreno R, Espejo JA, Cabrera A, Millan T, Gil J (2006) Ploidic and molecular analysis of ‘Morado de Huetor’ Asparagus(Asparagus officinalisL.) population: a Spanish Tetraploid Landrace. Genet Resour Crop Evol 53:729–736. https://doi.org/10.1007/s10722-004-4717-0
Moreno R, Espejo JA, Cabrera A, Gil J (2008a) Origin of tetraploid cultivated asparagus landraces inferred from nrDNA ITS polymorphisms. Ann Appl Biol 153:233–241. https://doi.org/10.1111/j.1744-348.2008.00254.x
Moreno R, Espejo JA, Moreno MT, Gil J (2008b) Collection and conservation of ‘Morado de Huetor’ Spanish tetraploid asparagus landrace. Genet Resour Crop Evol 55:773–777. https://doi.org/10.1007/s10722-008-9358-2
Murashige T, Skoog F (1962) A revised medium for rapid growth and bioassays with tobacco tissue culture. Physiol Plant 15:473–497
Nishizawa S, Sakai A, Amano Y, Matsuzawa T (1992) Cryopreservation of Asparagus(Asparagus officinalisL.) embryogenic cells and subsequent plant regeneration by a simple freezing method. Cryo Lett 13:379–388
Nishizawa S, Sakai A, Amano Y, Matsuzawa T (1993) Cryopreservation of Asparagus(Asparagus officinalisL.) embryogenic suspension cells and subsequent plant regeneration by vitrification. Plant Sci 91:67–73
Odake Y, Udagawa A, Saga H, Mii M (1993) Somatic embryogenesis of tetraploid plants from intermodal segments of a diploid cultivar of Asparagus officinalisL. grown in liquid culture. Plant Sci 94:173–177
Ozaki Y, Narikiyo K, Fujita C, Okubo H (2004) Ploidy variation of progenies from intra- and inter-ploidy crosses with regard to trisomic production in asparagus (Asparagus officinalisL.). Sex Plant Reprod 17:157–164
Panis B, Lambardi M (2005) Status of cryopreservation technologies in plants (crops and forest trees). In: The role of biotechnology for the characterization and conservation of crop, forest,animal and fishery genetic resources in developing countries. FAO, Turin, Italy, pp 43–54
Pontaroli AC, Camadro EL (2005) Somaclonal variation in Asparagus officinalisL. plants regenerated by organogenesis from long-term callus cultures. Genet Mol Biol 28:423–430
Raimondi JP, Camadro EL, Masuelli RW (2001) Assesment of somaclonal variation in Asparagusby RAPD fingerprinting and cytogenetic analyses. Sci Hortic 90:19–29
Redenbaugh K, Paasch BD, Nichol JW, Kossler ME, Viss PR, Walker KA (1986) Somatic Seeds: Encapsulation of Asexual plant Embryos. Nat Biotechnol 4:791–801
Reed BM (1996) Pretreatment strategies for the cryopreservation of plant tissues. In: Normah MN, Narimah MK, Clyde NM (eds) In Vitroconservation of plant genetic resources. Plant Biotechnology Laboratory, Faculty of Life Sciences, University Kebangsaan, Bangi
Regalado JJ, Carmona-Martín E, Castro P, Moreno R, Gil J, Encina CL (2015a) Micropropagation of wild species of the genus AsparagusL. and their interspecific hybrids with cultivated A. officinalisL., and verification of genetic stability using EST-SSRs. Plant Cell Tissue Organ Cult 121:501–510
Regalado JJ, Carmona-Martín E, Moreno R, Gil J, Encina CL (2015b) Study of the somaclonal variation produced by different methods of polyploidization with colchicine in Asparagus officinalisl. Plant Cell Tissue Organ Cult 122:31–44
Saha S, Sengupta C, Ghosh P (2015) Encapsulation, short-term storage, conservation and molecular analysis to assess genetic stability in alginate-encapsulate microshoots of Ocimum kilimandscharicumGuerke. Plant Cell Tissue Organ Cult 120:519–530
Sakai A, Matsumoto T, Hirai D, Niino T (2000) Newly developed encapsulation-dehydration protocol for plant cryopreservation. Cryo Lett 21:53–62
Suzuki T, Kaneko M, Harada T (1997) Increase in freezing resistance of excised shoots tips of Asparagus officinalisL. by preculture on sugar-rich media. Cryobiology 34:264–275
Suzuki T, Kaneko M, Harada T, Yakuwa T (1998) Enhanced formation of roots and subsequent promotion of growth of shoots on cryopreserved nodal segments of Asparagus officinalisL. Cryobiology 36:194–205
Torres AM, Weeden NF, Martin A (1993) Linkage among isozyme, RFLP and RAPD markers in Vicia Faba. Theor Appl Genet 85:935–945
Uragami A, Sakai A, Nagai M (1990) Cryopreservation of dried axillary buds plantlets of Asparagus officinalisL. grown in vitro. Plant Cell Rep 9:328–331
Wang R, Gao X, Chen L, Huo L, Li M (2014) Shoot recovery and genetic integrity of Chrysantemum morifolium shoot tips following cryopreservation by droplet-method. Sci Hortic 176 (2014):330–339
Yang HJ, Cloré WJ (1974) Development of complet plantlets from moderately vigorous shoot of stocks of Asparagusin vitro. HortScience 9:138–139
Author information
Authors and Affiliations
Contributions
All authors conceived and planned the experiments. E.C.M. performed the experiments and wrote the manuscript with input from all authors.
Corresponding author
Ethics declarations
Conflict of interest
The authors declare that the research review was conducted in the abscense of any commercial or financial relationships that could be construed as a potential conflict of interest.
Additional information
Communicated by Sergio J. Ochatt.
Rights and permissions
About this article
Cite this article
Carmona-Martín, E., Regalado, J.J., Perán-Quesada, R. et al. Cryopreservation of rhizome buds of Asparagus officinalis L. (cv. Morado de Huétor) and evaluation of their genetic stability. Plant Cell Tiss Organ Cult 133, 395–403 (2018). https://doi.org/10.1007/s11240-018-1392-y
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11240-018-1392-y