Abstract
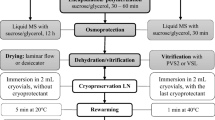
A vitrification method enabled efficient cryopreservation of embryogenic tissue (ETs) of Norway spruce (Picea abies L.) at −196 °C in liquid nitrogen (LN). Correctly formed, normal somatic embryos were generated from ETs that had been thawed after removal from LN. The pregrowth-dehydration method involved preculture of ETs with sucrose (0.25–1.00 M) in the presence or absence of 10 μM abscisic acid (ABA), followed by air-drying for 2 h and rapid freezing in LN. Pretreatment of ETs with both sucrose and ABA promoted ET growth after preculture and thawing more effectively than treatment with sucrose alone. Survival of ETs after thawing from LN using both sucrose and ABA was 54.4 % compared to pretreatment with sucrose alone which was 20 %. Addition of ABA in the preculture medium also improved the ability of ETs to form cotyledonary stage somatic embryos. The somatic embryos, which had normal shoot and root apices and the correct number of cotyledons, were indistinguishable from regenerants obtained from control cultures. Genetic analysis of control and cryopreserved ETs, as well as somatic embryos derived from cryopreserved ETs, indicated that the cryopreservation method had no effect on any of the five microsatellite loci (SpAGC1, SpAGC2, SpAGG3, SpAC1H8, and SpAC1F7) tested. The cryopreservation protocol outlined should enable the long-term storage of valuable clones of Norway spruce in LN, potentially for hundreds of years.








Similar content being viewed by others
References
Aronen TS, Krajnakova J, Häggman HM, Ryynänen LA (1999) Genetic fidelity of cryopreserved embryogenic cultures of open-pollinated Abies cephalonica. Plant Sci 142:163–172
Burg K, Hristoforoglu K, Fluch S, Hohl A, Burg A, Schmidt J (1999) Analysis of the fidelity of DNA replication in embryogenic cultures of Norway spruce. In: Espinel S, Ritter E (eds) Proceedings of application of biotechnology to forest genetics. Biofor 99 Vitoria-Gasteiz, Spain, pp 231–235
Burg K, Helmersson A, Bozhkov P, von Arnold S (2007) Developmental and genetic variation in nuclear microsatellite stability during somatic embryogenesis in pine. J Exp Bot 58:687–698
Chmielarz P (2009a) Cryopreservation of dormant European ash (Fraxinus excelsior) orthodox seeds. Tree Physiol 29:1279–1285
Chmielarz P (2009b) Cryopreservation of dormant orthodox seeds of forest trees: mazzard cherry (Prunus avium L.). Ann Forest Sci 66:405p1–405p9
Chmielarz P, Grenier-de March G, de Boucaud MT (2005) Cryopreservation of Quercus robur L. embryogenic calli. Cryoletters 26:349–355
Chmielarz P, Michalak M, Pałucka M, Wasileńczyk U (2011) Successful cryopreservation of Quercus robur plumules. Plant Cell Rep 30:1405–1414
Cloutier D, Rioux D, Beaulieu J, Schoen DJ (2003) Somatic stability of microsatellite loci in Eastern white pine, Pinus strobus L. Heredity 90:247–252
Cyr DR, Lazaroff WR, Grimes SMA, Quan G, Bethune TD, Dunstan DI, Roberts DR (1994) Cryopreservation of interior spruce (Picea glauca engelmanni complex) embryogenic cultures. Plant Cell Rep 13:574–577
DeVerno LL, Park YS, Bonga JM, Barrett JD (1999) Somaclonal variation in cryopreserved embryogenic clones of white spruce (Picea glauca (Moench) Voss.). Plant Cell Rep 18:948–953
Engelmann F (2004) Cryopreservation: progress and prospects. In Vitro Cell Dev Biol Plant 40:427–433
Engelmann F, Engles J, Dullo E (2003) The development of complementary strategies for the conservation of plant genetic resources using in vitro and cryopreservation methods. In: Chaudhury R, Pandey R, Malik SK, Mal B (eds) In vitro conservation and cryopreservation of tropical fruit species. IPGRI Office for South Asia and NBPGR, New Delhi, pp 37–48
Fang JY, Sacandé M, Pritchard H, Wetten A (2009) Influence of freezable/non-freezable water and sucrose on the viability of Theobroma cacao somatic embryos following desiccation and freezing. Plant Cell Rep 28:883–889
Fernandes P, Rodriguez E, Pinto G, Roldán-Ruiz I, De Loose M, Santos C (2008) Cryopreservation of Quercus suber somatic embryos by encapsulation-dehydration and evaluation of genetic stability. Tree Physiol 28:1841–1850
Fourré JL, Berger P, Niquet L, André P (1997) Somatic embryogenesis and somaclonal variation in Norway spruce: morphogenetic, cytogenetic and molecular approaches. Theor Appl Genet 94:159–169
Gale S, John A, Harding K, Benson EE (2008) Developing cryopreservation for Picea sitchensis (Sitka spruce) somatic embryos: a comparison of vitrification protocols. Cryoletters 29:135–144
Glaubitz JC, Moran GF (2000) Genetic tools: the use of biochemical and molecular markers. In: Young A, Boshier D, Boyle T (eds) Forest conservation genetics: principles and practice. CSIRO Publishing, Collingwood, pp 39–59
Grenier-de March G, de Boucaud MT, Chmielarz P (2005) Cryopreservation of Prunus avium L. embryogenic tissues. Cryoletters 26:341–348
Gusta LV, Trischuk R, Weiser CJ (2005) Plant cold acclimation: the role of abscisic acid. J Plant Growth Regul 24:308–318
Häggman HM, Ryynänen LA, Aronen TS, Krajnakova J (1998) Cryopreservation of embryogenic cultures of Scots pine. Plant Cell, Tissue Organ Cult 54:45–53
Harding K (2004) Genetic integrity of cryopreserved plant cells: a review. Cryoletters 25:3–22
Harvengt L, Trontin JF, Reymont I, Canlet F, Paques M (2001) Molecular evidence of true-to-type propagation of 3-year old Norway spruce through somatic embryogenesis. Planta 213:828–832
Hazubska-Przybył T, Bojarczuk K (2008) Somatic embryogenesis of selected spruce species (Picea abies, P. omorika, P. pungens ‘Glauca’ and P. breweriana). Acta Soc Bot Pol 77:89–199
Hazubska-Przybył T, Chmielarz P, Michalak M, Bojarczuk K (2010) Cryopreservation of embryogenic tissues of Picea omorika (Serbian spruce). Plant Cell, Tissue Organ Cult 102:35–44
Heinz B, Schmidt J (1995) Monitoring genetic fidelity vs somaclonal variation in Norway Spruce (Picea abies) somatic embryogenesis by RAPD analysis. Euphytica 85:341–345
Helmersson A, Von Arnold S, Burg K, Bozhkov PV (2004) High stability of nuclear microsatellite loci during the early stages of somatic embryogenesis in Norway spruce. Tree Physiol 24:1181–1186
Helmersson A, Jansson G, Bozhkov PV, Von Arnold S (2008) Genetic variation in microsatellite stability of somatic embryo plants of Picea abies: a case study using six unrelated full-sib families. Scand J Forest Res 23:2–11
Isabel N, Boivin R, Levasseur C, Charest PM, Bousquet J, Tremblay FM (1996) Occurrence of somaclonal variation among somatic embryo-derived white spruces (Picea glauca. Pinaceae). Am J Bot 83:1121–1130
Jitsuyama Y, Suzuki T, Harada T, Fujikawa S (2002) Sucrose incubation increases freezing tolerance of Asparagus (Asparagus officinalis L.) embryogenic cell suspensions. Cryoletters 23:103–112
Kendall EJ, Kartha KK, Qureshi JA, Chermak P (1993) Cryopreservation of immature spring wheat zygotic embryos using an abscisic acid pretreatment. Plant Cell Rep 12:89–94
Kim HM, Shin JH, Sohn JK (2006) Cryopreservation of somatic embryos of the herbaceous peony (Peonia lactiflora Pall.) by air drying. Cryobiology 53:69–74
Klimaszewska K, Ward C, Cheliak WM (1992) Cryopreservation and plant regeneration from embryogenic cultures of larch (Larix x eurolepis) and black spruce (Picea mariana). J Exp Bot 43:73–79
Kong L, Attree SM, Evans DE, Binarova P, Yeung EC, Fowke LC (1999) Somatic embryogenesis in white spruce: studies of embryo development and cell biology. In: Jain SM, Gupta PK, Pramod PK, Newton RJ (eds) Somatic embryogenesis in woody plants, vol 4. Kluwer Academic Publishers, Dordrecht, The Netherlands, pp 1–28
Kunkel TA, Benek R (2000) DNA replication fidelity. Annu Rev Biochem 69:497–529
Label P, Lelu MA (2000) Exogenous abscisic acid fate during maturation of hybrid larch (Larix x leptoeuropaea) somatic embryos. Physiol Plant 109:456–462
Lambardi M, Ozudogru A, Benelli C (2008) Cryopreservation of embryogenic cultures. In: Reed BM (ed) Plant Cryopreservation: a practical guide. Springer, USA, pp 177–194
Lian C, Oishi R, Miyashita N, Hogetsu T (2004) Hight somatic instability of a microsatellite locus in a clonal tree Robinia pseudoacacia. Theor Appl Genet 108:836–841
Litvay JD, Verma DC, Johnson MA (1985) Influence of loblolly pine (Pinus taeda L.) culture medium and its components on growth and somatic embryogenesis of wild carrot (Daucus carrota L.). Plant Cell Rep 4:325–328
Lopes T, Pinto G, Loureiro J, Costa A, Santos C (2006) Determination of genetic stability in long-term somatic embryogenic cultures and derived plantlets of cork oak using microsatellite markers. Tree Physiol 26:1145–1152
Malabadi RB, Nataraja K (2006) Cryopreservation and plant regeneration via somatic embryogenesis using shoot apical domes of mature Pinus roxburghii Sarg. Trees. In Vitro Cell Dev Biol Plant 42:152–159
Martínez MT, Ballester A, Vieitez AM (2003) Cryopreservation of embryogenic cultures of Quercus robur using desiccation and vitrification procedures. Cryobiology 46:182–189
Marum L, Rocheta M, Maroco J, Oliveira MM, Miguel C (2009) Analysis of genetic stability at SSR loci during somatic embryogenesis in maritime pine (Pinus pinaster). Plant Cell Rep 28:673–682
Mikuła A (2008) Krioprezerwacja jako metoda zachowania zdolności życiowych komórek i tkanek roślinnych [Cryopreservation as a method to maintain the life processes of cells and plant tissues]. Biotechnologia 2:41–57
Misra S (1994) Conifer zygotic embryogenesis, somatic embryogenesis and seed germination: biochemical and molecular advances. Seed Sci Res 4:357–384
Park YS, Barret JD, Bonga M (1998) Application of somatic embryogenesis in high: value clonal forestry: deployment, genetic control and stability of cryopreserved clones. In Vitro Cell Dev Biol 34:231–239
Pennycooke JC, Towill LE (2000) Cryopreservation of shoot tips from in vitro plants of sweet potato (Ipomoea batatas Lam.) by vitrification. Plant Cell Rep 19:733–739
Percy RE, Livingstone NJ, Moran JA, von Aderkas P (2001) Desiccation, cryopreservation and water relations parameters of white spruce (Picea glauca) and interior spruce (Picea glauca × engelmanniii complex) somatic embryos. Tree Physiol 2:1303–1310
Pfeiffer A, Olivieri AM, Morgante M (1997) Identification and characterization of microsatellites in Norway spruce (Picea abies K.). Genome 40:411–419
Popova EV (2010) Cryopreservation of coriander (Coriandrum sativum L.) somatic embryos using sucrose preculture and air desiccation. Sci Hort 124:522–528
Pritchard HW, Nadarajan J (2008) Cryopreservation of orthodox (desiccation tolerant) seeds. In: Reed BM (ed) Plant cryopreservation: a practical guide. Springer, LLC, pp 485–494
Rahman MH, Rajola OP (2001) Microsatellite DNA somaclonal variation in micropropagated trembling aspen (Populus tremuloides). Plant Cell Rep 20:531–536
Reaney MJT, Gusta LV (1987) Factors influencing the induction of freezing tolerance by abscisic acid in cell suspension cultures of Bromus inermis Leyss and Medicago sativa L. Plant Physiol 83:423–427
Reed BM (1993) Responses to ABA and cold acclimation are genotype dependent for cryopreserved black-berry and raspberry meristems. Cryobiology 30:179–184
Richards CM, Reilley A, Touchell D, Antolin MF, Walters C (2004) Microsatellite primers for Texas ild rice (Zizania texana) and preliminary test of the impact of cryogenic storage on allele frequency at these loci. Conserv Genet 5:853–859
Robertson AJ, Ishikawa M, Gusta LV, McKenzie S (1994) Abscisic acid-induced heat tolerance in Bromus inermis cell cultures. Plant Physiol 105:181–190
Ryynanen L (1998) Effect of abscisic acid, cold hardening and photoperiod on recovery of cryopreserved in vitro shoot tips of silver birch. Cryobiology 36:32–39
Sakai A (2000) Development of cryopreservation techniques. In: Engelmann F, Takagi H (eds) Cryopreservation of tropical plant germplasm. Current Research Progress and Applications, JIRCAS, IPGRI, Rome, pp 1–7
Smulders MJM, Rus-Kortekass W, Visman B (1995) Tissue culture-induced DNA methylation polymorphism in repetitive DANN of tomato calli and regenerated plants. Theor Appl Genet 91:1257–1264
Suzuki M, Ishikawa M, Akihama T (1998) A novel preculture metod for the induction of desiccation tolerance in gentian axillary buds for cryopreservation. Plant Sci 135:69–76
Suzuki M, Ishikawa M, Okuda H, Noda K, Kishimoto T, Nakamura T, Ogiwara I, Shimura I, Akihama T (2006) Physiological changes in gentian axillary buds during two-step preculturing with sucrose that conferred high levels of tolerance to desiccation and cryopreservation. Ann Bot 97:1073–1081
Thierry C, Tessereau H, Florin B, Meschine MC, Pétiard V (1997) Role of sucrose for the aquisition of tolerance to cryopreservation of carrot somatic embryos. Cryoletters 18:283–292
Touchell DH, Chiang VL, Tsai CJ (2002) Cryopreservation of embryogenic cultures of Picea mariana (black spruce) using vitrification. Plant Cell Rep 21:118–124
Verlaysen H, Samyn G, Van Bockstaele E, Debergh P (2004) Evaluation of analytical techniques to predict viability after cryopreservation. Plant Cell, Tissue Organ Cult 77:11–21
Walters C, Wesley-Smith J, Crane J, Hill LM, Chmielarz P, Pammenter N, Berjak P (2008) Cryopreservation of recalcitrant (i.e. desiccation-sensitive) seeds. In: Reed BM (ed) Plant cryopreservation: a practical guide. Springer, LLC, pp 465–484
Wang ZY, Legris G, Nagel J, Potrykus I, Spangenberg G (1994) Cryopreservation of embryogenic cell suspensions in Festuca and Lolium species. Plant Sci 103:93–106
Wilhelm E, Hristoforoglu K, Fluch S, Burg K (2005) Detection of microsatellite instability during somatic embryogenesis of oak (Quercus robur L.). Plant Cell Rep 23:790–795
Zhu GY, Geuns JMC, Dussert S, Swennen R, Panis B (2006) Change in sugar, sterol and fatty acid composition in banana meristems caused by sucrose-induced acclimation and its effect on cryopreservation. Physiol Plant 128:80–94
Acknowledgments
This study was supported by the National Science Centre in Krakow (Grant no. N N309 130837).
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Hazubska-Przybył, T., Chmielarz, P., Michalak, M. et al. Survival and genetic stability of Picea abies embryogenic cultures after cryopreservation using a pregrowth-dehydration method. Plant Cell Tiss Organ Cult 113, 303–313 (2013). https://doi.org/10.1007/s11240-012-0270-2
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11240-012-0270-2