Abstract
Early neurological deterioration (END) following intravenous recombinant tissue plasminogen activator (rt-PA) treatment is a serious clinical event that can be caused by hemorrhagic or ischemic insult. We investigated the differences in predictive factors for END due to hemorrhagic and END due to ischemic insults. Consecutive patients from four hospitals who received 0.6 mg/kg intravenous rt-PA for acute ischemic stroke were retrospectively recruited. END was defined as a National Institutes of Health Stroke Scale (NIHSS) score ≥ 4 points within 24 h compared with baseline. END was classified into those due to hemorrhagic (ENDh) or ischemic (ENDi) insult based on computed tomography (CT) or magnetic resonance imaging. Risk factors associated with ENDh and ENDi were investigated by comparison with non-END cases. A total of 744 patients (452 men, median 75 years old) were included. END was observed in 79 patients (10.6%), including 22 ENDh (3.0%) and 57 ENDi (7.7%), which occurred within a median of 7 h after treatment. Multivariate analyses showed that higher pretreatment NIHSS score (odds ratio [OR] 1.06, 95% confidence interval [CI] 1.00–1.13) and pretreatment with antiplatelets (OR 2.84, 95% CI 1.08–7.72) were associated with ENDh. Extensive early ischemic change (Alberta Stroke Program Early CT Score ≤ 7 on CT or ≤ 6 on diffusion-weighted imaging; OR 2.80, 95% CI 1.36–5.64) and large artery occlusions (OR 3.09, 95% CI 1.53–6.57) were associated with ENDi. Distinct factors were predictive for the END subtypes. These findings could help develop preventative measures for END in patients with the identified risk factors.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Highlights
-
Early neurological deterioration (END) following intravenous recombinant tissue plasminogen activator (rt-PA) for acute ischemic stroke is a serious clinical event; nevertheless, its etiology has not been well defined.
-
Risk factors associated with END following intravenous rt-PA due to hemorrhagic (ENDh) or ischemic insults (ENDi) were investigated by comparing with non-END cases.
-
Severe stroke symptoms and pretreatment with antiplatelets were associated with ENDh, and large-sized infarcts and large artery occlusions were associated with ENDi.
-
Identification of these differences in predictive factors for END subtypes could inform the development of preventative measures for END following intravenous rt-PA.
Introduction
Intravenous recombinant tissue plasminogen activator (rt-PA) is an effective treatment for acute ischemic stroke. However, early neurological deterioration (END), classically defined as any ≥ 4 points increase on the National Institutes of Health Stroke Scale (NIHSS) score within 24 h compared with baseline [1], occurs in more than 10% of patients who received intravenous rt-PA [2]. END leads to high mortality and poor functional outcomes [2, 3]. The primary causes of END have been reported to be symptomatic intracranial hemorrhage (sICH), malignant edema [2, 4], and early recurrent ischemic stroke (ERIS), the occurrence of new neurological symptoms involving initially unaffected vascular territories, and evidence of corresponding ischemic lesions [5, 6]. Other reported cases exhibit unexplained neurological deterioration; this is presumably caused by an infarct growth beyond the initial penumbra, which is often referred to as ‘stroke progression’ [7,8,9]. Previous studies have demonstrated that several clinical and radiological factors on admission and/or at 24 h were associated with END [1, 9,10,11,12,13,14,15]. While the detailed cause for deterioration has rarely been identified, there might be differences between the risk factors for END due to ischemic insults and those for END due to hemorrhagic insults. Indeed, a post hoc analysis of data from one randomized clinical trial showed a significant association between early addition of aspirin after intravenous rt-PA and END due to sICH but not due to cerebral ischemia [16]. Given this context, we hypothesized that there are different predictive factors for END due to ischemic insults and END due to hemorrhagic insults. Clarifying the risk factors for END subtypes might aid the development of preventative measures for END following intravenous rt-PA. Therefore, the aim of this study was to investigate differences in predictive factors for END due to hemorrhagic insults and END due to ischemic insults.
Methods
Subjects
We used data from a multicenter retrospective observational study, which has been previously described in detail [17]. Briefly, this study was conducted with patients from the stroke unit of four urban emergency hospitals (Saiseikai Fukuoka General Hospital, Fukuoka City Hospital, Iizuka Hospital, and Kokura Memorial Hospital). The subjects of this study were consecutive patients who received intravenous rt-PA for acute ischemic stroke between October 1st, 2005 and December 31st, 2015. All patients received intravenous administration of 0.6 mg/kg alteplase in accordance with the Japanese guidelines [18]. This study was approved by the ethics committees of Kyushu University Hospital (29-111) and those of each of the four facilities. Written informed consent was waived because of the retrospective study design.
The following clinical information was systematically collected from medical records: age, sex, vascular risk factors (hypertension, diabetes mellitus, and dyslipidemia), atrial fibrillation (AF), previous history of stroke, and pretreatment with antiplatelets and anticoagulants. Severity of stroke symptoms was assessed by the NIHSS score, which was obtained before the administration of rt-PA. Computed tomography (CT) and/or magnetic resonance imaging (MRI) including diffusion-weighted imaging (DWI) were performed prior to administration of rt-PA for the assessment of early ischemic change (EIC). At least two experienced physicians retrospectively evaluate EIC using the Alberta Stroke Program Early CT Score (ASPECTS) [19, 20] on CT and/or DWI in each facility, without using a central reading system. Extensive EIC was defined as an ASPECTS of ≤ 7 on CT or ≤ 6 on DWI. Arterial occlusion sites were assessed by MR angiography, carotid ultrasonography, and/or CT angiography. Large artery occlusions were defined as one or more occlusions of the internal carotid artery (ICA), proximal portion of the middle cerebral artery (MCA), and/or the basilar artery (BA) detected by any modality. The pretreatment systolic and diastolic blood pressure and glycemia, and onset-to-needle time were obtained from emergency medical charts. Endovascular therapy alongside thrombolysis was performed for eligible patients, including mechanical clot disruption and retrieval, and angioplasty with or without stenting.
Definition of END and subtypes
After the treatment, patients’ symptoms were closely monitored. END was defined as a neurological deterioration with a ≥ 4 points increase on the NIHSS score compared with baseline within 24 h after the administration of rt-PA. The time of END onset was collected from medical records. Brain imaging was principally performed at the time of deterioration and/or 24 h after the treatment. Each END case was retrospectively reviewed and classified into those due to hemorrhagic (ENDh) or ischemic (ENDi) insults as follows: ENDh is an END presumably caused by a parenchymal intracerebral hematoma, which refers to a hematoma with a mass effect occupying 30% or more of the infarct in the ischemic region (Fig. 1a) or subarachnoid hemorrhage; ENDi is an END other than ENDh, and which includes malignant edema, ERIS, and unexplained neurological deterioration (Fig. 1b–d). Patients whose symptoms deteriorated as a result of other clinically apparent causes were excluded.
Representative brain imaging of patients with early neurological deterioration due to hemorrhagic (a) and ischemic (b–d) insults. a Axial computed tomography (CT) performed 8 h after thrombolysis showed an extended parenchymal hematoma of the right basal ganglia with additional blood in both lateral ventricles and hydrocephalus. b Axial CT performed 18 h after thrombolysis showed extensive brain edema and midline shift. c Axial CT performed 12 h after thrombolysis in a patient who became comatose during thrombolysis for ischemic stroke in the left middle cerebral artery (MCA) territory showed new acute ischemic lesions in the right MCA territory. c Diffusion-weighted imaging obtained 12 h after thrombolysis showed a hyperintense lesion in the territory of lenticulostriate arteries, which did not change much from baseline
Statistical analysis
All statistical analyses were performed using JMP statistical software version 9.0 (SAS Institute Inc., Cary, NC, USA). Data are expressed as medians and interquartile ranges for continuous variables and counts and percentages for categorical variables. Clinical characteristics were compared using the Chi squared test, Fisher’s exact test, or Wilcoxon rank sum test as appropriate. We then performed multivariate logistic regression analyses including variables that were significantly associated with ENDh and ENDi in the univariate analysis to identify factors associated with both ENDh and ENDi. A p-value of < 0.05 was considered statistically significant.
Results
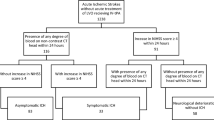
A total 750 patients that had received intravenous rt-PA were registered during the study period. A flowchart of patient selection is shown in Fig. 2. Among these, 6 patients were excluded because they had received intravenous rt-PA twice during the study period (n = 2), exhibited a deterioration of symptoms due to factors other than stroke (cardiac arrest due to ventricular arrhythmia [n = 2] and intubation due to heart failure [n = 1]), or missing data (n = 1). Finally, 744 patients (452 men, median 75 years old) were included in the analysis. Antiplatelets were prescribed to 204 patients (27.4%) prior to stroke, including aspirin (n = 167), clopidogrel (n = 45), and/or others (n = 25). Thirty-three patients (4.4%) received dual antiplatelet therapy. Anticoagulants were prescribed to 121 patients (16.3%), including warfarin (n = 109), direct oral anticoagulants (n = 8), or unfractionated heparin (n = 4). Before thrombolysis, CT was performed in 651 patients (87.5%) and 54 patients (8.3%) were classified as having an ASPECTS ≤ 7; MRI was performed in 617 patients (82.9%) and 79 patients (12.8%) were classified as having an ASPECTS ≤ 6 on DWI. As a result, 99 out of the 744 patients (13.3%) were classified as having extensive EIC. Large artery occlusions were seen in 306 patients (47.8%) out of 640 patients who were evaluated for arterial occlusion sites before thrombolysis, and these included ICA (n = 102), proximal portion of MCA (n = 165), and BA (n = 39). Endovascular therapy alongside thrombolysis was received by 82 patients (26.8%) with large artery occlusions. END was seen in 79 patients (10.6%) including 22 ENDh (3.0%) and 57 ENDi (7.7%). END occurred a median of 7 h after the initiation of treatment (8 [1.75–17.25] hours in ENDh and 4 [1.0–13.5] hours in ENDi, p = 0.147), and most of them occurred within the first 2 h.
Univariate analyses of clinical characteristics between non-END patients and patients with ENDh and ENDi are shown in Table 1. Compared with non-END patients, the extensive EIC and large artery occlusions were more common in patients with both END subtypes; the pretreatment NIHSS score was higher (20.5 [16–26] vs. 14 [8–20], p = 0.001) and pretreatment with antiplatelets was more common (50.0% vs. 27.7%, p = 0.022) in patients with ENDh, and AF was more common in patients with ENDi (64.9% vs. 49.3%, p = 0.027). The multivariate analyses revealed that a higher pretreatment NIHSS score (odds ratio [OR] 1.06, 95% confidence interval [CI] 1.00–1.13) and pretreatment with antiplatelets (OR 2.84, 95% CI 1.08–7.72) were associated with ENDh. Eleven patients with antiplatelet pretreatment developed ENDh, and all of them had taken aspirin (9 patients with aspirin only, 1 with dual antiplatelet therapy, and 1 with aspirin and warfarin). Extensive EIC (OR 2.80, 95% CI 1.36–5.64) and large artery occlusions (OR 3.09, 95% CI 1.53–6.57) were associated with ENDi.
Discussion
This study found that END occurred in approximately one-tenth of patients who received intravenous rt-PA for acute ischemic stroke. Among them, sICH accounted for one-third of the overall cause of END. This frequency and proportion of END was comparable to previous studies in which 0.9 mg/kg alteplase was administrated [1, 2, 7, 9, 13, 15]. The median time from administration of rt-PA to deterioration was 8 h for ENDh and 4 h for ENDi, and mainly occurred within first 2 h. Similar results were reported in a recent study in which END occurred at a mean of 7.3 h after rt-PA administration in patients with sICH, and 4.8 h in those without [15]. Moreover, ERIS has been reported to occur during or shortly after rt-PA administration [5, 6]. These data indicate that END occurs early after the administration of rt-PA, regardless of hemorrhagic or ischemic insults.
This study identified some distinct factors that were predictive for END subtypes; severe stroke symptoms and pretreatment with antiplatelets were associated with ENDh, and extensive EIC and large artery occlusions were associated with ENDi. Similar to previous studies [21,22,23], a higher pretreatment NIHSS score was associated with ENDh. In the Japan Alteplase Clinical Trial [24], 5 of 6 patients who developed sICH had a pretreatment NIHSS score of ≥ 19. These results conflict with the reported association between END due to ischemia and lower baseline NIHSS scores, which were suggestive of good collateral flow at baseline [3, 7]. Imaging studies have demonstrated that lower residual cerebral blood flow and lower apparent diffusion coefficient value in ischemic lesions were predictive of sICH [25, 26]. Taken together, a higher NIHSS score rather than a lower ASPECTS might be suggestive of a greater depth of ischemia with irreversible tissue damage, which leads an increased risk of hemorrhage on reperfusion.
We found a significant association between pretreatment with antiplatelets and ENDh. Although the association between pretreatment with antiplatelets and overall END has not been reported in previous studies, this result was similar to that of early addition of aspirin after intravenous rt-PA and END due to sICH [16]. This may help to predict which patients are prone to ENDh and may also inform early post-thrombolytic management (e.g. stricter blood pressure control and restricted use of nonsteroidal anti-inflammatory drugs within the initial 24 h).
An extensive EIC, defined as an ASPECTS of ≤ 7 on CT or ≤ 6 on DWI, represents a large-sized infarct or multiple acute cerebral infarcts. Krieger et al. [27] demonstrated that a hypodensity of > 50% of the MCA territory on initial CT predicts fatal brain swelling. An ASPECTS of ≤ 7 on CT has been associated with extensive EIC in the one-third MCA territory method [28]. Thus, extensive EIC representing a large-sized infarct in the MCA territory might be associated with a high risk for END due to brain edema. Multiple acute cerebral infarcts are another cause of the extensive EIC, and presumably derive from symptomatic ICA stenosis/occlusion or cardiac embolism [29], which are known predictors of ENDi [3, 6, 9].
Large artery occlusions were surrogate markers for not only the penumbra, but also the oligemia. In a previous report from Seners et al. [7], alongside the proximal occlusion of large arteries, no-recanalization was a predictor for END due to causes other than sICH or malignant edema. The secondary hemodynamic or metabolic disruption of the oligemic tissue mainly due to the loss of collateral flow has been suggested to be one of the major mechanisms of unexplained neurological deterioration [7,8,9]. In this study, only 26.8% of patients with large artery occlusions underwent endovascular therapy adjacent to thrombolysis. This finding suggests that the reduction in the incidence of ENDi is one of the therapeutic effects of the recent mechanical thrombectomy alongside thrombolysis in patients with acute ischemic stroke and large artery occlusions.
This study has several limitations. First, this study had a retrospective design with a limited number of patients and facilities, which could lead to some selection bias and statistical errors. Second, we did not study some pre- and post-treatment parameters that could potentially affect the incidence of END (e.g. time from taking antithrombotic agents to the treatment, blood pressure variability, no recanalization, and arterial reocclusion). Third, ischemic lesions in the posterior circulation might be overlooked by the ASPECTS. Fourth, the associations between END subtype and clinical outcome were unclear, because the 3-month modified Rankin Scale was not investigated in the present study. Finally, some END related to endovascular procedures might be included, because patients received endovascular treatment alongside thrombolysis were not excluded.
In conclusion, END occurred in approximately one-tenth of patients receiving intravenous rt-PA, and ENDi was three times more common than ENDh. Distinct factors were associated with END subtypes. Our findings might inform the development of preventative measures for END following intravenous rt-PA in patients with these risk factors.
References
Grotta JC, Welch KM, Fagan SC et al (2001) Clinical deterioration following improvement in the NINDS rt-PA stroke trial. Stroke 32:661–668
Seners P, Turc G, Oppenheim C et al (2015) Incidence, causes and predictors of neurological deterioration occurring within 24 h following acute ischaemic stroke: a systematic review with pathophysiological implications. J Neurol Neurosurg Psychiatry 86:87–94
Mori M, Naganuma M, Okada Y et al (2012) Early neurological deterioration within 24 hours after intravenous rt-PA therapy for stroke patients: the stroke acute management with urgent risk factor assessment and improvement rt-PA registry. Cerebrovasc Dis 34:140–146
Siegler JE, Martin-Schild S (2011) Early neurological deterioration (END) after stroke: the END depends on the definition. Int J Stroke 6:211–212
Georgiadis D, Engelter S, Tettenborn B et al (2006) Early recurrent ischemic stroke in stroke patients undergoing intravenous thrombolysis. Circulation 114:237–241
Awadh M, MacDougall N, Santosh C et al (2010) Early recurrent ischemic stroke complicating intravenous thrombolysis for stroke: incidence and association with atrial fibrillation. Stroke 41:1990–1995
Seners P, Turc G, Tisserand M et al (2014) Unexplained early neurological deterioration after intravenous thrombolysis: incidence, predictors, and associated factors. Stroke 45:2004–2009
Tisserand M, Seners P, Turc G et al (2014) Mechanisms of unexplained neurological deterioration after intravenous thrombolysis. Stroke 45:3527–3534
Simonsen CZ, Schmitz ML, Madsen MH et al (2016) Early neurological deterioration after thrombolysis: clinical and imaging predictors. Int J Stroke 11:776–782
Alvarez-Sabín J, Molina CA, Montaner J et al (2003) Effects of admission hyperglycemia on stroke outcome in reperfused tissue plasminogen activator–treated patients. Stroke 34:1235–1241
Rubiera M, Ribo M, Delgado-Mederos R et al (2006) Tandem internal carotid artery/middle cerebral artery occlusion: an independent predictor of poor outcome after systemic thrombolysis. Stroke 37:2301–2305
Topakian R, Strasak AM, Nussbaumer K et al (2008) Prognostic value of admission C-reactive protein in stroke patients undergoing iv thrombolysis. J Neruol 255:1190–1196
Aries MJ, Uyttenboogaart M, Koopman K et al (2009) Hyperdense middle cerebral artery sign and outcome after intravenous thrombolysis for acute ischemic stroke. J Neurol Sci 285:114–117
Haeusler KG, Gerischer LM, Vatankhah B et al (2011) Impact of hospital admission during nonworking hours on patient outcomes after thrombolysis for stroke. Stroke 42:2521–2525
James B, Chang AD, McTaggart RA et al (2018) Predictors of symptomatic intracranial haemorrhage in patients with an ischaemic stroke with neurological deterioration after intravenous thrombolysis. J Neurol Neurosurg Psychiatry 89:866–869
Zinkstok SM, Beenen LF, Majoie CB et al (2014) Early deterioration after thrombolysis plus aspirin in acute stroke: a post hoc analysis of the antiplatelet therapy in combination with recombinant t-PA thrombolysis in ischemic stroke trial. Stroke 45:3080–3082
Tanaka K, Matsumoto S, Yamada T et al (2019) Temporal trends in clinical characteristics and door-to-needle time in patients receiving intravenous tissue plasminogen activator: a retrospective study of 4 hospitals in Japan. J Stroke Cerebrovasc Dis 28:104305
Minematsu K, Toyoda K, Hirano T et al (2013) Guidelines for the intravenous application of recombinant tissue-type plasminogen activator (alteplase), the second edition, October 2012: a guideline from the Japan Stroke Society. J Stroke Cerebrovasc Dis 22:571–600
Barber PA, Demchuk AM, Zhang J et al (2000) Validity and reliability of a quantitative computed tomography score in predicting outcome of hyperacute stroke before thrombolytic therapy: ASPECTS Study Group Alberta Stroke Programme Early CT Score. Lancet 355:1670–1674
Nezu T, Koga M, Nakagawara J et al (2011) Early ischemic change on CT versus diffusion-weighted imaging for patients with stroke receiving intravenous recombinant tissue-type plasminogen activator therapy: stroke acute management with urgent risk-factor assessment and improvement (SAMURAI) rt-PA registry. Stroke 42:2196–2200
Saver JL (2007) Hemorrhage after thrombolytic therapy for stroke: the clinically relevant number needed to harm. Stroke 38:2279–2283
Strbian D, Sairanen T, Meretoja A et al (2011) Patient outcomes from symptomatic intracerebral hemorrhage after stroke thrombolysis. Neurology 77:341–348
Lokeskrawee T, Muengtaweepongsa S, Patumanond J et al (2017) Prediction of symptomatic intracranial hemorrhage after intravenous thrombolysis in acute ischemic stroke: the symptomatic intracranial hemorrhage score. J Stroke Cerebrovasc Dis 26:2622–2629
Yamaguchi T, Mori E, Minematsu K et al (2006) Alteplase at 0.6 mg/kg for acute ischemic stroke within 3 hours of onset: Japan Alteplase Clinical Trial (J-ACT). Stroke 37:1810–1815
Ueda T, Hatakeyama T, Kumon Y et al (1994) Evaluation of risk of hemorrhagic transformation in local intra-arterial thrombolysis in acute ischemic stroke by initial SPECT. Stroke 25:298–303
Shinoda N, Hori S, Mikami K et al (2017) Prediction of hemorrhagic transformation after acute thrombolysis following major artery occlusion using relative ADC ratio: a retrospective study. J Neuroradiol 44:361–366
Krieger DW, Demchuk AM, Kasner S et al (1999) Early clinical and radiological predictors of fatal brain swelling in ischemic stroke. Stroke 30:287–292
Mak HK, Yau KK, Khong PL et al (2003) Hypodensity of > 1/3 middle cerebral artery territory versus Alberta Stroke Programme Early CT Score (ASPECTS): comparison of two methods of quantitative evaluation of early CT changes in hyperacute ischemic stroke in the community setting. Stroke 34:1194–1196
Novotny V, Thomassen L, Waje-Andreassen U et al (2017) Acute cerebral infarcts in multiple arterial territories associated with cardioembolism. Acta Neurol Scand 135:346–351
Acknowledgments
We thank the Center for Clinical and Translational Research, Kyushu University, for the maintenance and management of the Research Electronic Data Capture database. We thank Dr. Chie Kikutake, Medical Institutes of Bioregulation, Kyushu University, for advice on statistical analyses. We also thank Nia Cason, PhD, from Edanz Group (www.edanzediting.com/ac) for editing a draft of this manuscript.
Funding
This study was supported by the Japan Society for the Promotion of Science Grant-in-Aid for Scientific Research, Grant Number 16K10727 and 19H01045, Grant-in-Aid for Research Activity start-up, Grant Number 19K21303, and Grant-in-Aid for Challenging Research (Pioneering), Grant number 19H05562.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interests
The authors declare no conflicts of interest.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/.
About this article
Cite this article
Tanaka, K., Matsumoto, S., Furuta, K. et al. Differences between predictive factors for early neurological deterioration due to hemorrhagic and ischemic insults following intravenous recombinant tissue plasminogen activator. J Thromb Thrombolysis 49, 545–550 (2020). https://doi.org/10.1007/s11239-019-02015-4
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11239-019-02015-4