Abstract
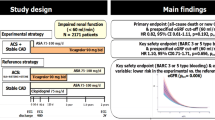
Patients with renal disease are often undertreated with antiplatelet therapy due to concerns about bleeding. Vorapaxar blocks platelet activation via the PAR-1 receptor and reduces cardiovascular events in patients with stable atherosclerosis, but with increased bleeding. We examined the efficacy and safety of vorapaxar in patients with impaired renal function. TRA2°P-TIMI 50 randomized patients with stable atherosclerosis to vorapaxar or. We analyzed patients with eGFR assessed who qualified with a history of MI or PAD (without stroke or TIA) (n = 19,932). Cox models assessed the risk of CV events and bleeding by quartile of baseline eGFR in the placebo arm and then by randomized assignment. Net clinical outcome (NCO) was predefined as CV death, MI, stroke, or GUSTO severe bleeding. Patients with lower eGFR tended to be older, female, have hypertension, hyperlipidemia or prior PAD. In the placebo arm, baseline eGFR in the lowest quartile was associated with a 26% higher risk of CV death, MI or stroke (Q1:Q4 HRadj 1.26, 1.03–1.55) and 73% higher risk of GUSTO moderate or severe bleeding (HRadj 1.73, 1.12–2.65). Vorapaxar reduced the risk of MACE to a similar extent (14–26%) across quartiles of baseline eGFR (P interaction = 0.70) and increased the relative risk of GUSTO moderate or severe bleeding (P interaction = 0.54). NCO was similar across quartiles of eGFR (P interaction = 0.65). Intensification of antiplatelet therapy with vorapaxar offers comparable net clinical benefit regardless of baseline renal function. These data support the use of more potent antiplatelet regimens in patients with renal dysfunction.



Similar content being viewed by others
References
Sarnak MJ, Levey AS, Schoolwerth AC, Coresh J, Culleton B, Hamm LL, McCullough PA, Kasiske BL, Kelepouris E, Klag MJ, Parfrey P, Pfeffer M, Raij L, Spinosa DJ, Wilson PW (2003) Kidney disease as a risk factor for development of cardiovascular disease: a statement from the American Heart Association Councils on kidney in cardiovascular disease, high blood pressure research, clinical cardiology, and epidemiology and prevention. Circulation 108(17):2154–2169. https://doi.org/10.1161/01.cir.0000095676.90936.80
Clase CM, Gao P, Tobe SW, McQueen MJ, Grosshennig A, Teo KK, Yusuf S, Mann JF (2011) Estimated glomerular filtration rate and albuminuria as predictors of outcomes in patients with high cardiovascular risk: a cohort study. Ann Intern Med 154(5):310–318. https://doi.org/10.7326/0003-4819-154-5-201103010-00005
Shlipak MG, Heidenreich PA, Noguchi H, Chertow GM, Browner WS, McClellan MB (2002) Association of renal insufficiency with treatment and outcomes after myocardial infarction in elderly patients. Ann Intern Med 137(7):555–562
Wright RS, Reeder GS, Herzog CA, Albright RC, Williams BA, Dvorak DL, Miller WL, Murphy JG, Kopecky SL, Jaffe AS (2002) Acute myocardial infarction and renal dysfunction: a high-risk combination. Ann Intern Med 137(7):563–570
Best PJ, Lennon R, Ting HH, Bell MR, Rihal CS, Holmes DR, Berger PB (2002) The impact of renal insufficiency on clinical outcomes in patients undergoing percutaneous coronary interventions. J Am Coll Cardiol 39(7):1113–1119
Kahn MR, Robbins MJ, Kim MC, Fuster V (2013) Management of cardiovascular disease in patients with kidney disease. Nat Rev Cardiol 10(5):261–273. https://doi.org/10.1038/nrcardio.2013.15
Han JH, Chandra A, Mulgund J, Roe MT, Peterson ED, Szczech LA, Patel U, Ohman EM, Lindsell CJ, Gibler WB (2006) Chronic kidney disease in patients with non-ST-segment elevation acute coronary syndromes. Am J Med 119(3):248–254. https://doi.org/10.1016/j.amjmed.2005.08.057
Ocak G, Rookmaaker MB, Algra A, de Borst GJ, Doevendans PA, Kappelle LJ, Verhaar MC, Visseren FL (2018) Chronic kidney disease and bleeding risk in patients at high cardiovascular risk: a cohort study. J Thromb Haemost 16(1):65–73. https://doi.org/10.1111/jth.13904
Ishigami J, Grams ME, Naik RP, Coresh J, Matsushita K (2016) Chronic kidney disease and risk for gastrointestinal bleeding in the community: the atherosclerosis risk in communities (ARIC) study. Clin J Am Soc Nephrol 11(10):1735–1743. https://doi.org/10.2215/cjn.02170216
Melgaard L, Overvad TF, Skjoth F, Christensen JH, Larsen TB, Lip GYH (2018) Risk of stroke and bleeding in patients with heart failure and chronic kidney disease: a nationwide cohort study. ESC Heart Fail 5(2):319–326. https://doi.org/10.1002/ehf2.12256
Melloni C, Cornel JH, Hafley G, Neely ML, Clemmensen P, Zamoryakhin D, Prabhakaran D, White HD, Fox KA, Ohman EM, Armstrong PW, Roe MT (2016) Impact of chronic kidney disease on long-term ischemic and bleeding outcomes in medically managed patients with acute coronary syndromes: insights from the TRILOGY ACS trial. Eur Heart J Acute Cardiovasc Care 5(6):443–454. https://doi.org/10.1177/2048872615598631
Morrow DA, Braunwald E, Bonaca MP, Ameriso SF, Dalby AJ, Fish MP, Fox KA, Lipka LJ, Liu X, Nicolau JC, Ophuis AJ, Paolasso E, Scirica BM, Spinar J, Theroux P, Wiviott SD, Strony J, Murphy SA (2012) Vorapaxar in the secondary prevention of atherothrombotic events. N Engl J Med 366(15):1404–1413. https://doi.org/10.1056/NEJMoa1200933
Morrow DA, Scirica BM, Fox KA, Berman G, Strony J, Veltri E, Bonaca MP, Fish P, McCabe CH, Braunwald E (2009) Evaluation of a novel antiplatelet agent for secondary prevention in patients with a history of atherosclerotic disease: design and rationale for the thrombin-receptor antagonist in secondary prevention of atherothrombotic ischemic events (TRA 2 degrees P)–TIMI 50 trial. Am Heart J 158(3):335–341.e333. https://doi.org/10.1016/j.ahj.2009.06.027
Magnani G, Bonaca MP, Braunwald E, Dalby AJ, Fox KA, Murphy SA, Nicolau JC, Oude Ophuis T, Scirica BM, Spinar J, Theroux P, Morrow DA (2015) Efficacy and safety of vorapaxar as approved for clinical use in the United States. J Am Heart Assoc 4(3):e001505. https://doi.org/10.1161/jaha.114.001505
Administration USFaD (Revised: 05/2014) ZONTIVITY Highlights of Prescribing Information. https://www.accessdata.fda.gov/drugsatfda_docs/label/2014/204886s000lbl.pdf
Levey AS, Bosch JP, Lewis JB, Greene T, Rogers N, Roth D (1999) A more accurate method to estimate glomerular filtration rate from serum creatinine: a new prediction equation. Modification of diet in renal disease study group. Ann Intern Med 130(6):461–470
Go AS, Chertow GM, Fan D, McCulloch CE, Hsu C-y (2004) Chronic kidney disease and the risks of death, cardiovascular events, and hospitalization. N Engl J Med 351(13):1296–1305. https://doi.org/10.1056/NEJMoa041031
Adams MJ, Irish AB, Watts GF, Oostryck R, Dogra GK (2008) Hypercoagulability in chronic kidney disease is associated with coagulation activation but not endothelial function. Thromb Res 123(2):374–380. https://doi.org/10.1016/j.thromres.2008.03.024
Sagripanti A, Cozza V, Baicchi U, Camici M, Cupisti A, Barsotti G (1997) Increased thrombin generation in patients with chronic renal failure. Int J Clin Lab Res 27(1):72–75
van Gorp RH, Schurgers LJ (2015) New insights into the pros and cons of the clinical use of vitamin k antagonists (VKAs) versus direct oral anticoagulants (DOACs). Nutrients 7(11):9538–9557. https://doi.org/10.3390/nu7115479
Borissoff JI, Spronk HMH, ten Cate H (2011) The hemostatic system as a modulator of atherosclerosis. N Engl J Med 364(18):1746–1760. https://doi.org/10.1056/NEJMra1011670
Kosoglou T, Kraft WK, Kumar B, Statkevich P, Xuan F, Ma L, Jennings LK, Schiller JE, Langdon RB, Cutler DL (2012) Pharmacokinetics and pharmacodynamics of the novel PAR-1 antagonist vorapaxar in patients with end-stage renal disease. Eur J Clin Pharmacol 68(7):1049–1056. https://doi.org/10.1007/s00228-012-1217-6
Keltai M, Tonelli M, Mann JF, Sitkei E, Lewis BS, Hawken S, Mehta SR, Yusuf S (2007) Renal function and outcomes in acute coronary syndrome: impact of clopidogrel. Eur J Cardiovasc Prev Rehabil 14(2):312–318. https://doi.org/10.1097/01.hjr.0000220582.19516.a6
Wiviott SD, Braunwald E, McCabe CH, Montalescot G, Ruzyllo W, Gottlieb S, Neumann FJ, Ardissino D, De Servi S, Murphy SA, Riesmeyer J, Weerakkody G, Gibson CM, Antman EM (2007) Prasugrel versus clopidogrel in patients with acute coronary syndromes. N Engl J Med 357(20):2001–2015. https://doi.org/10.1056/NEJMoa0706482
James S, Budaj A, Aylward P, Buck KK, Cannon CP, Cornel JH, Harrington RA, Horrow J, Katus H, Keltai M, Lewis BS, Parikh K, Storey RF, Szummer K, Wojdyla D, Wallentin L (2010) Ticagrelor versus clopidogrel in acute coronary syndromes in relation to renal function: results from the Platelet Inhibition and Patient Outcomes (PLATO) trial. Circulation 122(11):1056–1067. https://doi.org/10.1161/circulationaha.109.933796
Magnani G, Storey RF, Steg G, Bhatt DL, Cohen M, Kuder J, Im K, Aylward P, Ardissino D, Isaza D, Parkhomenko A, Goudev AR, Dellborg M, Kontny F, Corbalan R, Medina F, Jensen EC, Held P, Braunwald E, Sabatine MS, Bonaca MP (2016) Efficacy and safety of ticagrelor for long-term secondary prevention of atherothrombotic events in relation to renal function: insights from the PEGASUS-TIMI 54 trial. Eur Heart J 37(4):400–408. https://doi.org/10.1093/eurheartj/ehv482
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
Dr. Bonaca reports grant support from Amgen, AstraZeneca, Merck, MedImmune and Pfizer; and receipt of consulting fees from Amgen, Aralez, AstraZeneca, Bayer, Janssen, Merck and Sanofi. Dr. Scirica reports research grants via Brigham and Women’s Hospital from AstraZeneca, Eisai, Novartis, and Merck; consulting fees from AstraZeneca, Biogen Idec, Boehringer Ingelheim, Covance, Dr. Reddy’s Laboratory, Eisai, Elsevier Practice Update Cardiology, GlaxoSmithKline, Lexicon, Merck, NovoNordisk, Sanofi, St. Jude’s Medical; and equity in Health [at] Scale. Dr. Morrow reports receipt of consulting fees from Abbott Laboratories, Aralez, AstraZeneca, DiaDexus, GlaxoSmithKline, Merck and Company, Peloton, Roche Diagnostics, Verseon; and research grants from Abbott, Amgen, AstraZeneca, Daichii Sankyo Ltd, GlaxoSmithKline, Merck and Company, Pfizer, Novartis Pharmaceuticals, Roche Diagnostics. Dr. O’Donoghue reports research grants from GlaxoSmithKline, Eisai, AstraZeneca, Merck, Janssen, The Medicines Company. Dr Simon Correa, Erica Goodrich and Sabina Murphy have nothing to disclose.
Ethical approval
All procedures performed in studies involving human participants were in accordance with the ethical standards of the institutional and/or national research committee and with the 1964 Helsinki declaration and its later amendments or comparable ethical standards.
Research involving human participants and/or animals and Informed consent
In the TRA2°P-TIMI 50 trial, informed consent was obtained from all individual participants included in the study.
Electronic supplementary material
Below is the link to the electronic supplementary material.
Rights and permissions
About this article
Cite this article
Correa, S., Bonaca, M.P., Scirica, B.M. et al. Efficacy and safety of more potent antiplatelet therapy with vorapaxar in patients with impaired renal function. J Thromb Thrombolysis 47, 353–360 (2019). https://doi.org/10.1007/s11239-018-1779-y
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11239-018-1779-y