Abstract
The Nobel Prize of Physiology or Medicine 2023 was awarded jointly to two researchers, Katalin Karikó and Drew Weissman, for their discoveries taking part in the development of effective mRNA vaccines against COVID-19. The accelerated development of the novel mRNA-lipid nanoparticle vaccines provided highly effective protection against severe disease or death, and reduction in symptomatic illness, before a full year had passed from the beginning of the pandemic.
Similar content being viewed by others
Nobel recognition
The Nobel Prize 2023 in Physiology or Medicine has been awarded jointly to Katalin Karikó and Drew Weissman, for their discoveries concerning nucleoside base modifications that allow the development of effective mRNA vaccines against COVID-19 [1].
Their discoveries were significant for developing effective mRNA vaccines against COVID-19 during the pandemic that began in the early 2020. Their innovative findings changed the understanding of how mRNA interacts with our immune system and therefore played an important role in the rapid rate of vaccine development during the COVID-19 pandemic, which is one of the unexpected extraordinary dangers to human health in the XXI century [1].
The never expected danger of human in the XXI century—the COVID-19
At the end of 2019, a new coronavirus appeared in Wuhan, China, causing severe respiratory syndrome and pneumonia with very high mortality. On 11 March 2020, the World Health Organization (WHO) specified it as a pandemic, since it has caused extensive morbidity and mortality around the world [2, 3].
On 19 December 2023, there have been 772,838,745 confirmed cases of COVID-19, including 6,988,679 deaths, reported to the WHO [4].
SARS-CoV-2 is an enveloped virus with positive-sense single-stranded RNA that possesses the largest genomes (~ 30 kb) among the known RNA viruses, belonging to the betacoronavirus genus of the family Coronaviridae [2].
SARS-CoV-2 like other RNA viruses easily undergo mutation very frequently (about million times higher mutation rate then their host) causing appearance of new variants that differ in characteristics with the original strain and resulting in virulence and adaptability, therefore responsible for multiple episodes of the pandemic, and questioning the vaccines’ effectiveness. The main explanation for its global spread is mutations linked to the spike protein, crucial for the attachment and virus entry in host cells via angiotensin-converting enzyme receptor 2 [2, 3].
The development of varied vaccines against the COVID-19 has played an important role in controlling this life-threatening disease. The vaccines have two categories: one is based on viral components, and another is based on the whole virus. The four most important structural proteins of the coronavirus, envelope, membrane and spike proteins found on the viral surface envelope, and nucleocapsid protein, present in the ribonucleoprotein core are the primary targets for potential vaccines [2].
An efficient vaccine must be easy to develop, reproduce and administer, safe, thermostable and with low manufacturing cost. Over 200 COVID-19 vaccine researches are in progress among which a large number of vaccines with a variety of platforms have been given approval for clinical trials [2] (Fig. 1).
COVID-19 vaccine platforms [2]
Vaccines
Vaccines helps the immune system to develop immunity from a disease, containing a microorganism or virus in a weakened, live or killed state, or proteins or toxins from the organism. The stimulating the organ’s adaptive immunity, helps to prevent sickness from the infectious disease in the event of a later exposure. Vaccines based on killed or weakened viruses have long been available, e.g., vaccines against polio, measles, and yellow fever. In 1951, a South-American virologist, physician Max Theiler was awarded the Nobel Prize in Physiology or Medicine for developing the yellow fever vaccine in 1937, using weakened virus as the result passing the virus through laboratory mice. Between 1940 and 1947, the produced more than 28 million doses of the vaccine ended yellow fever as a major disease [1, 5].
The World Health Organization (WHO) estimates that 2–3 million lives are saved each year by current immunization programs, contributing to the marked reduction in mortality of children less than 5 years of age globally from 93 deaths per 1000 live births in 1990 to 39 deaths per 1000 live births in 2018 [6].
The introduction of vaccination against infectious diseases such as diphtheria (part a), capsular group C meningococcus, polio, Haemophilus influenzae type B, measles, and pertussis led to a significant decrease in their incidence. For pertussis, a decline in vaccine coverage led to an increase in cases in the late 1970s and 1980s, but disease incidence reduced again after vaccine coverage increased [6].
Later, vaccines based on individual viral components (especially proteins found on the virus surface), rather than whole viruses, have been developed (e.g., vaccines against the hepatitis B virus and human papillomavirus). Alternatively, parts of the viral genetic code can be moved to a harmless carrier virus, a “vector,” used in vaccines against the Ebola virus. When vector vaccines are injected, the selected viral protein is produced, stimulating an immune response against the targeted virus [1].
Producing whole virus-, protein-, and vector-based vaccines requires large-scale cell culture. This resource-intensive process limits the possibilities for rapid vaccine production in response to outbreaks and pandemics. Therefore, researchers have long attempted to develop vaccine technologies independent of cell culture [1] (Table 1.)
mRNA vaccines
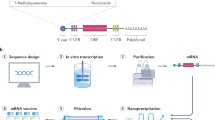
The DNA-encoded genetic information is transferred to messenger RNA (mRNA), which is used as a template for protein synthesis in the cells. mRNA was discovered in 1961; in vitro transcription was introduced in 1984 made it possible to generate any desired mRNA from the encoding plasmid using phage RNA polymerases. The first successful demonstration of mRNA delivery to cells using liposomes was provided in 1989. Ideas of using mRNA technologies for vaccine and therapeutic purposes also arose, because of simplified cell-free manufacturing, cell cycle-independent performance, and no risk of insertional mutagenesis. In 1990, for the first time, the therapeutic potential of mRNA was shown, but because in vitro transcribed mRNA is considered to be unstable and challenging to be delivered, requiring the development of carrier lipid systems to encapsulate the mRNA and the risk of inflammatory reactions, the developing the mRNA technology was initially constricted [1, 7,8,9].
Karikó and Weissman’s roaring discovery
The Hungarian biochemist Katalin Karikó, despite the difficulties, remained to investigate mRNA as therapeutic options. With her colleague, Drew Weissman, at the University of Pennsylvania, they were focusing on how different RNA types interact with the immune system.
They noticed that dendritic cells, having important functions in immune surveillance and the activation of vaccine-induced immune responses, recognize in vitro transcribed mRNA, which leads to their activation and the release of inflammatory signaling molecules via Toll-like receptors. However, mRNA from the mammalian cells did not give the same reactions. Karikó and Weissman’s explanation was that mammalian cell originating mRNA bases are frequently methylated or otherwise modified, in comparison with in vitro transcribed mRNA, resulting in unwanted cytokine release and inflammatory reaction in dendritic cells. They demonstrated that most potent RNAs activating immune cells are those that had the least number of modified nucleosides. They hypothesized that nucleoside modification suppresses the immune-stimulatory effect of RNA. These results were published in 2005, fifteen years before the COVID-19 pandemic [1, 10].
Further, they proved and published in 2008 and 2010, that base-modified mRNAs result in reduced inflammatory responses and increased protein production [11, 12]. Replacing uridine with pseudouridine made the mRNA non-immunogenic, more stable and highly translatable [9].
In vitro transcribed mRNA has many advantages as a vehicle for gene delivery. Transfection of mRNA is very efficient, with the rapid expression of the encoded protein. Cell-delivered mRNA does not increase the risk of insertional mutagenesis, vs. viral vectors or plasmid DNA. The potential of mRNA as a delivery vehicle is enhanced further by incorporating modified nucleosides that reduce host defense responses [12].
In 2010, several companies were working on developing the method. Vaccines against Zika virus and MERS-CoV were looked for. After the outbreak of the COVID-19 pandemic, base-modified mRNA vaccines encoding the SARS-CoV-2 surface protein were developed at record speed. Protective effects of around 95% were reported; vaccines were approved as early as December 2020 [1].
mRNA-based vaccines use genetically engineered mRNA to exercise the host cells to express antigenic viral proteins against which the immune system produces antibodies that protect the host body in case of later infections with the same virus. The use of mRNA in the development of a vaccine against COVID-19 is a novel approach. The major advantage of mRNA vaccines is their flexibility and effectiveness. The Food and Drug Administration (FDA) currently endorsed three mRNA-based vaccinations against COVID-19 with efficacy of about 72% to 95% in trials against moderate-to-severe COVID-19 among adults [2].
Regarding mRNA constructed vaccine, the mRNA is assimilated inside lipid nanoparticles to promote its delivery into host cells and prevent degradation. After vaccination, the lipid nanoparticles enclosing mRNA enter host cells, and then the lipid layer is gradually degraded, and mRNA is released and becomes available for cellular translational machinery to produce its corresponding viral protein, which can elicit both cellular and humoral immunity after trafficking to the plasma membrane [13].
As of 9 December 2023, a total of 13 617 649 012 vaccine doses have been administered. (https://covid19.who.int/). The vaccines have saved millions of lives and prevented severe disease and have also decreased COVID-19-related hospitalization, deaths, and prevention of ICU admission. Controllable spread of SARS-CoV-2 can be accomplished earlier when a large proportion of the population is immunized (e.g., 70–80%). Immunization significantly reduces the incidence of COVID-19 [4, 14].
The mRNA vaccines created the basis for vaccines against other infectious diseases. As a transient carrier of genetic information, the in vitro transcribed mRNA (encoding tumor antigens, cytokines, and tumor suppressors) provides anticancer therapy by enabling the production of encoded proteins without genomic integration [7].
Recently, mRNA-constructed vaccines have been considered an appropriate replacement for traditional vaccines due to their high efficiency, safety, low investment, and fast production. However, their production and utilization are limited due to low stability, ultra-freezing formulation and sometimes a failure of mRNA delivery into the targeted human cell [13]. Thereafter, there is a need of more research to improve the delivery efficiency of mRNA to targeted organs and tissues (for example, spleen, brain, lung, lymph node, and kidney). The administration routes of mRNA also should be expanded to include the possibility of oral delivery, dissolvable microneedle patch for vaccination, and noninvasive aerosol inhalation [15].
Katalin Karikó and Drew Weissman’s discoveries regarding nucleoside-based modifications allowed the development of effective mRNA vaccines against COVID-19. So finally, it took 60 years until the first mRNA became an FDA-approved product in the form of COVID-19 mRNA vaccine.
Data availability
No datasets were generated or analyzed during the current study.
References
https://www.nobelprize.org/prizes/medicine/2023. Accessed 24 Jan 2024
Yadav T, Kumar S, Mishra G, Saxena SK (2023) Tracking the COVID-19 vaccines: the global landscape. Hum Vaccin Immunother 19:2191577
Malik JA, Ahmed S, Mir A, Shinde M, Bender O, Alshammari F, Ansari M, Anwar S (2022) The SARS-CoV-2 mutations versus vaccine effectiveness: new opportunities to new challenges. J Infect Public Health 15:228–240
https://covid19.who.int. Accessed 24 Jan 2024
Frierson JG (2010) The yellow fever vaccine: a history. Yale J Biol Med 8:77–85
Pollard AJ, Bijker EM (2021) A guide to vaccinology: from basic principles to new developments. Nat Rev Immunol 21:83–100
Karikó K (2019) In vitro-transcribed mRNA therapeutics: out of the shadows and into the spotlight. Mol Ther 27:691–692
Trepotec Z, Lichtenegger E, Plank C, Aneja MK, Rudolph C (2019) Delivery of mRNA Therapeutics for the treatment of hepatic diseases. Mol Ther 27:794–802
Karikó K (2022) Developing mRNA for Therapy. Keio J Med 71:31
Karikó K, Buckstein M, Ni H, Weissman D (2005) Suppression of RNA recognition by toll-like receptors: the impact of nucleoside modification and the evolutionary origin of RNA. Immunity 23:165–175
Karikó K, Muramatsu H, Welsh FA, Ludwig J, Kato H, Akira S, Weissman D (2008) Incorporation of pseudouridine into mRNA yields superior nonimmunogenic vector with increased translational capacity and biological stability. Mol Ther 16:1833–1840
Anderson BR, Muramatsu H, Nallagatla SR, Bevilacqua PC, Sansing LH, Weissman D, Karikó K (2010) Incorporation of pseudouridine into mRNA enhances translation by diminishing PKR activation. Nucleic Acids Res 38:5884–5892
Abulsoud AI, El-Husseiny HM, El-Husseiny AA, El-Mahdy HA, Ismail A, Elkhawaga SY, Khidr EG, Fathi D, Mady EA, Najda A, Algahtani M, Theyab A, Alsharif KF, Albrakati A, Bayram R, Abdel-Daim MM, Doghish AS (2023) Mutations in SARS-CoV-2: Insights on structure, variants, vaccines, and biomedical interventions. Biomed Pharmacother 157:113977
Chavda VP, Jogi G, Dave S, Patel BM, Vineela Nalla L, Koradia K (2023) mRNA-based vaccine for COVID-19: they are new but not unknown! Vaccines (Basel) 11:507
Liu C, Shi Q, Huang X, Koo S, Kong N, Tao W (2023) mRNA-based cancer therapeutics. Nat Rev Cancer 23:526–543
Funding
Open access funding provided by Semmelweis University. The Open access is funded by the Semmelweis University.
Author information
Authors and Affiliations
Contributions
H.K. wrote the manuscript.
Corresponding author
Ethics declarations
Competing interests
The authors declares no competing interests.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/.
About this article
Cite this article
Hagymási, K. The breakthrough in vaccination: Nobel Prize in Physiology or Medicine 2023. Struct Chem 35, 695–699 (2024). https://doi.org/10.1007/s11224-024-02281-w
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11224-024-02281-w