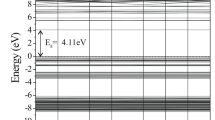
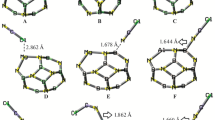
Abstract
Fullerene-like nanocages are being highly investigated in recent times for gas sensing applications. In this work, we explored the hydrogen selenide (H2Se) gas adsorption on the surfaces of pure and Ni-doped fullerene-like Al12N12 (AlN), Al12P12 (AlP), B12N12 (BN), and B12P12 (BP) nanocages using density functional theory (DFT). The interaction of H2Se gas with BN, BP, and Ni-doped BN nanocages exhibit low adsorption energy, whereas AlN, AlP, and Ni-decorated B12P12 (Ni_BP) exhibit higher adsorption energy. To obtain better insight into the interaction of H2Se gas with the nanocages; dipole moment, HOMO-LUMO orbitals, NBO charge transfer, global indices, thermodynamic parameters, DOS, and UV spectrum are examined. The QTAIM analysis is also performed to know more about the microscopic insight of sensing behavior and the intermolecular bonding nature. Thus, our calculated results indicate that AlN, AlP, and Ni-doped nanocages are promising candidates for sensing of H2Se gas.








Similar content being viewed by others
Data availability
The data used to support the findings of this study are available from the corresponding author upon request.
Code availability
Gaussian 09 package program.
References
Salih E, Ayesh AI (2020) First principle investigation of H2Se, H2Te and PH3 sensing based on graphene oxide. Phys Lett A 384:126775. https://doi.org/10.1016/j.physleta.2020.126775
Xin F, Tian Y, Zhang X (2020) Ratiometric fluorescent probe for highly selective detection of gaseous H2Se. Dye Pigment 177:108274. https://doi.org/10.1016/j.dyepig.2020.108274
Abrishamifar SM, Heidari N, Razavi R et al (2018) The Cl functionalized aluminum nitride (AlN) and aluminum phosphide (AlP) nanocone sheets as hydrogen selenide (H2Se) sensor: a density functional investigation. Acta Chim Slov 65:208–212
The National Institute for Occupational Safety and Health, occupational health guideline for hydrogen selenide. https://www.cdc.gov/niosh/docs/81-123/pdfs/0336.
Colin A at AE H2Se gas detector, hydrogen selenium, selenium hydride...en.gasdetect.com. https://en.gazdetect.com/gas-information/h2se-gas-detector/
Aiga S Asia Industrial Gases Association. Code of practice hydrogen selenide H 2 Se. https://www.asiaiga.org/
William CLY, Yaws CL (2001) Hydrogen selenide, Matheson gas data book, 7 th ed., 2001, Matheson, basking ridge, NJ 07920. https://www.mathesongas.com. In: Matheson gas data book. Parsippany, NJ : Matheson Tri-Gas ; New York : McGraw-Hill.
Hussain S, Shahid Chatha SA, Hussain AI et al (2020) Designing novel Zn-decorated inorganic B12P12 nanoclusters with promising electronic properties: a step forward toward efficient CO2 sensing materials. ACS Omega 5:15547–15556. https://doi.org/10.1021/acsomega.0c01686
Ammar HY, Eid KM, Badran HM (2021) The impact of an external electric field on methanol adsorption on XB11N12 (X=B, Co, Ni) nano-cages: a DFT and TD-DFT study. J Phys Chem Solids 153:110033. https://doi.org/10.1016/j.jpcs.2021.110033
Shokuhi Rad A, Esfahanian M, Maleki S, Gharati G (2016) Application of carbon nanostructures toward SO2 and SO3 adsorption: a comparison between pristine graphene and N-doped graphene by DFT calculations. J Sulfur Chem 37:176–188. https://doi.org/10.1080/17415993.2015.1116536
Rad AS, Shadravan A, Soleymani AA, Motaghedi N (2015) Lewis acid-base surface interaction of some boron compounds with N-doped graphene; first principles study. Curr Appl Phys 15:1271–1277. https://doi.org/10.1016/j.cap.2015.07.018
Lv R, Li Q, Botello-Méndez AR et al (2012) Nitrogen-doped graphene: beyond single substitution and enhanced molecular sensing. Sci Rep 2:586. https://doi.org/10.1038/srep00586
Rad AS, Shabestari SS, Mohseni S, Aghouzi SA (2016) Study on the adsorption properties of O3, SO2, and SO3 on B-doped graphene using DFT calculations. J Solid State Chem 237:204–210. https://doi.org/10.1016/j.jssc.2016.02.023
Cui H, Zhang G, Zhang X, Tang J (2019) Rh-doped MoSe(2) as a toxic gas scavenger: a first-principles study. Nanoscale Adv 1:772–780. https://doi.org/10.1039/c8na00233a
Cui H, Zhang X, Li Y et al (2019) First-principles insight into Ni-doped InN monolayer as a noxious gases scavenger. Appl Surf Sci 494:859–866. https://doi.org/10.1016/j.apsusc.2019.07.218
Cui H, Yan C, Jia P, Cao W (2020) Adsorption and sensing behaviors of SF6 decomposed species on Ni-doped C3N monolayer: a first-principles study. Appl Surf Sci 512:145759. https://doi.org/10.1016/j.apsusc.2020.145759
Cui H, Feng Z, Wang W et al (2022) Adsorption behavior of Pd-doped PtS2 monolayer upon SF6 decomposed species and the effect of applied electric field. IEEE Sens J 22:6764–6771. https://doi.org/10.1109/JSEN.2022.3154119
Zhang C (2012) First-principles study on electronic structures and magnetic properties of AlN nanosheets and nanoribbons. J Appl Phys 111:43702. https://doi.org/10.1063/1.3686144
Kandalam AK, Pandey R, Blanco MA et al (2000) First principles study of polyatomic clusters of AlN, GaN, and InN. 1. Structure, stability, vibrations, and ionization. J Phys Chem B 104:4361–4367. https://doi.org/10.1021/jp994308s
Costales A, Kandalam AK, Franco R, Pandey R (2002) Theoretical study of structural and vibrational properties of (AlP) n,(AlAs) n,(GaP) n,(GaAs) n,(InP) n, and (InAs) n clusters with n= 1, 2, 3. J Phys Chem B 106:1940–1944
Yong Y, Liu K, Song B et al (2012) Coalescence of BnNn fullerenes: a new pathway to produce boron nitride nanotubes with small diameter. Phys Lett A 376:1465–1467
Guldi DM, Illescas BM, Atienza CM et al (2009) Fullerene for organic electronics. Chem Soc Rev 38:1587–1597. https://doi.org/10.1039/B900402P
Rad AS, Ayub K (2017) Adsorption properties of acetylene and ethylene molecules onto pristine and nickel-decorated Al12N12 nanoclusters. Mater Chem Phys 194:337–344. https://doi.org/10.1016/j.matchemphys.2017.04.002
Rad AS, Ayub K (2017) O3 and SO2 sensing concept on extended surface of B12N12 nanocages modified by nickel decoration: a comprehensive DFT study. Solid State Sci 69:22–30. https://doi.org/10.1016/j.solidstatesciences.2017.05.007
Shokuhi Rad A, Ayub K (2016) A comparative density functional theory study of guanine chemisorption on Al12N12, Al12P12, B12N12, and B12P12 nano-cages. J Alloys Compd 672:161–169. https://doi.org/10.1016/j.jallcom.2016.02.139
Jensen F, Toftlund H (1993) Structure and stability of C24 and B12N12 isomers. Chem Phys Lett 201:89–96. https://doi.org/10.1016/0009-2614(93)85039-Q
Bezi Javan M, Soltani A, Tazikeh Lemeski E et al (2016) Interaction of B12N12 nano-cage with cysteine through various functionalities: a DFT study. Superlattices Microstruct 100:24–37. https://doi.org/10.1016/j.spmi.2016.08.035
Baei MT (2013) B12N12 sodalite like cage as potential sensor for hydrogen cyanide. Comput Theor Chem 1024:28–33. https://doi.org/10.1016/j.comptc.2013.09.018
Oku T, Nishiwaki A, Narita I (2004) Formation and atomic structure of B12N12 nanocage clusters studied by mass spectrometry and cluster calculation. Sci Technol Adv Mater 5:635–638. https://doi.org/10.1016/j.stam.2004.03.017
Rad AS, Ayub K (2018) How can nickel decoration affect H2 adsorption on B12P12 nano-heterostructures? J Mol Liq 255:168–175. https://doi.org/10.1016/j.molliq.2018.01.149
Beheshtian J, Kamfiroozi M, Bagheri Z (2012) Theoretical study of hydrogen adsorption on the B 12P 12 fullerene-like nanocluster. Comput Mater Sci - Comput Mater Sci. https://doi.org/10.1016/j.commatsci.2011.09.039
Shokuhi Rad A, Bagheri Novir S, Mohseni S et al (2017) A DFT study of O2 and Cl2 adsorption onto Al12N12 fullerene-like nanocluster. Heteroat Chem 28:e21396. https://doi.org/10.1002/hc.21396
Beheshtian J, Bagheri Z, Kamfiroozi M, Ahmadi A (2012) A comparative study on the B12N12, Al12N12, B12P12 and Al12P12 fullerene-like cages. J Mol Model 18:2653–2658. https://doi.org/10.1007/s00894-011-1286-y
Soltani A, Baei MT, Taghartapeh MR et al (2015) Phenol interaction with different nano-cages with and without an electric field: a DFT study. Struct Chem 26:685–693. https://doi.org/10.1007/s11224-014-0504-5
Peyghan AA, Soleymanabadi H (2015) Computational study on ammonia adsorption on the X 12 Y 12 nano-clusters (X= B, Al and Y= N, P). Curr Sci 1910–1914
Beheshtian J, Peyghan AA, Bagheri Z (2012) Selective function of Al12N12 nano-cage towards NO and CO molecules. Comput Mater Sci 62:71–74. https://doi.org/10.1016/j.commatsci.2012.05.041
Beheshtian J, Bagheri Z, Kamfiroozi M, Ahmadi A (2011) Toxic CO detection by B12N12 nanocluster. Microelectronics J 42:1400–1403. https://doi.org/10.1016/j.mejo.2011.10.010
Munsif S, Maria, Khan S et al (2018) Remarkable nonlinear optical response of alkali metal doped aluminum phosphide and boron phosphide nanoclusters. J Mol Liq 271:51–64. https://doi.org/10.1016/j.molliq.2018.08.121
Rad AS, Ayub K (2016) Ni adsorption on Al12P12 nano-cage: a DFT study. J Alloys Compd 678:317–324. https://doi.org/10.1016/jjallcom201603175
Rad AS, Ayub K (2016) Enhancement in hydrogen molecule adsorption on B12N12 nano-cluster by decoration of nickel. Int J Hydrogen Energy 41:22182–22191
Rad AS, Ayub K (2016) Coordination of nickel atoms with Al12X12 (X = N, P) nanocages enhances H2 adsorption: a surface study by DFT. Vacuum 133:70–80. https://doi.org/10.1016/j.vacuum.2016.08.017
Russo T V, Martin RL, Hay PJ (1994) Density functional calculations on first‐row transition metals. J Chem Phys 101:7729–7737. https://doi.org/10.1063/1.468265
Barone V, Cossi M (1998) Quantum calculation of molecular energies and energy gradients in solution by a conductor solvent model. J Phys Chem A 102:1995–2001. https://doi.org/10.1021/jp9716997
Becke AD (1992) Density-functional thermochemistry. I. The effect of the exchange-only gradient correction. J Chem Phys 96:2155–2160
Becke AD (1988) Density-functional exchange-energy approximation with correct asymptotic behavior. Phys Rev A, Gen Phys 38:3098–3100. https://doi.org/10.1103/physreva.38.3098
Lee C, Yang W, Parr RG (1988) Development of the Colle-Salvetti correlation-energy formula into a functional of the electron density. Phys Rev B Condens Matter 37:785–789. https://doi.org/10.1103/physrevb.37.785
Kazemi M, Rad AS (2017) Sulfur mustard gas adsorption on ZnO fullerene-like nanocage: quantum chemical calculations. Superlattices Microstruct 106:122–128. https://doi.org/10.1016/j.spmi.2017.03.046
O’Boyle NM, Tenderholt AL, Langner KM (2008) Cclib: a library for package-independent computational chemistry algorithms. J Comput Chem. https://doi.org/10.1002/jcc.20823
Hossain MR, Hasan MM, Nishat M et al (2021) DFT and QTAIM investigations of the adsorption of chlormethine anticancer drug on the exterior surface of pristine and transition metal functionalized boron nitride fullerene. J Mol Liq 323:114627
Oishi AA, Dhali P, Das A et al (2022) Study of the adsorption of chloropicrin on pure and Ga and Al doped B12N12: a comprehensive DFT and QTAIM investigation. Mol Simul. https://doi.org/10.1080/08927022.2022.2053121
Rakib Hossain M, Mehade Hasan M, Ud Daula Shamim S et al (2021) First-principles study of the adsorption of chlormethine anticancer drug on C24, B12N12 and B12C6N6 nanocages. Comput Theor Chem 1197:113156. https://doi.org/10.1016/j.comptc.2021.113156
T.A. Keith (2010) AIMAll (Version 16.05. 18), TK Gristmill Software, Overl. Park KS, USA. https://doi.org/10.1007/BF02708340
Hay PJ, Wadt WR (1985) Ab initio effective core potentials for molecular calculations. Potentials for the transition metal atoms Sc to Hg. J Chem Phys 82:270–283. https://doi.org/10.1063/1.448799
Shamim SUD, Hussain T, Hossian MR et al (2020) A DFT study on the geometrical structures, electronic, and spectroscopic properties of inverse sandwich monocyclic boron nanoclusters ConBm (n = 1.2; m = 6–8). J Mol Model 26:1–17. https://doi.org/10.1007/s00894-020-04419-z
Pearson RG (1988) Absolute electronegativity and hardness: application to inorganic chemistry. Inorg Chem 27:734–740. https://doi.org/10.1021/ic00277a030
Hasan MM, Bithe SA, Neher B, Ahmed F (2022) Polythiophene as a sensor model for chlorofluorocarbon, fluorine, and oxygen gas using DFT calculations. J Mol Model 28:59. https://doi.org/10.1007/s00894-022-05048-4
Hossain MR, Hasan MM, Ashrafi NE, Rahman, H, Rahman MS, Ahmed F, Ferdous T, Hossain MA (2021) Adsorption behaviour of metronidazole drug molecule on the surface of hydrogenated graphene, boron nitride and boron carbide nano. Phys E Low-dimensional Syst Nanostructures 126:114483. https://doi.org/10.1016/j.physe.2020.114483
Keith TA (2010) AIMAll (Version 10.07. 25). Overland Park, KS, USA: TK Gristmill Software
Ullah F, Irshad S, Khan S et al (2021) Nonlinear optical response of first-row transition metal doped Al12P12 nanoclusters; a first-principles study. J Phys Chem Solids 151:109914. https://doi.org/10.1016/j.jpcs.2020.109914
Hossain MR, Hasan MM, Ashrafi N-E- et al (2021) Adsorption behaviour of metronidazole drug molecule on the surface of hydrogenated graphene, boron nitride and boron carbide nanosheets in gaseous and aqueous medium: a comparative DFT and QTAIM insight. Phys E Low-dimensional Syst Nanostructures 126:114483. https://doi.org/10.1016/j.physe.2020.114483
Rad AS, Valipour P, Gholizade A, Mousavinezhad SE (2015) Interaction of SO2 and SO3 on terthiophene (as a model of polythiophene gas sensor): DFT calculations. Chem Phys Lett 639:29–35
Shokuhi Rad A, Ayub K (2016) Adsorption of pyrrole on Al12N12, Al12P12, B12N12, and B12P12 fullerene-like nano-cages; a first principles study. Vacuum 131:135–141. https://doi.org/10.1016/j.vacuum.2016.06.012
Rad A, Kashani O (2015) Adsorption of acetyl halide molecules on the surface of pristine and Al-doped graphene: Ab initio study. Appl Surf Sci 355:233. https://doi.org/10.1016/j.apsusc.2015.07.113
Soltani A, Tazikeh-Lemeski E, Javan MB (2020) A comparative theoretical study on the interaction of pure and carbon atom substituted boron nitride fullerenes with ifosfamide drug. J Mol Liq 297:111894. https://doi.org/10.1016/j.molliq.2019.111894
Kamel M, Raissi H, Morsali A (2017) Theoretical study of solvent and co-solvent effects on the interaction of flutamide anticancer drug with carbon nanotube as a drug delivery system. J Mol Liq 248:490–500. https://doi.org/10.1016/j.molliq.2017.10.078
Carson EM, Watson JR (2002) Undergraduate students’ understandings of entropy and Gibbs free energy. Univ Chem Educ 6:4–12
Badran HM, Eid KM, Ammar HY (2021) DFT and TD-DFT studies of halogens adsorption on cobalt-doped porphyrin: effect of the external electric field. Results Phys 23:103964. https://doi.org/10.1016/j.rinp.2021.103964
Sajid H, Khan S, Ayub K, Mahmood T (2020) High selectivity of cyclic tetrapyrrole over tetrafuran and tetrathiophene toward toxic chemicals; a first-principles study. Microporous Mesoporous Mater. https://doi.org/10.1016/j.micromeso.2020.110126
Rozas I, Alkorta I, Elguero J (2000) Behavior of ylides containing N, O, and C atoms as hydrogen bond acceptors. J Am Chem Soc 122:11154–11161. https://doi.org/10.1021/ja0017864
Acknowledgements
We would like to thank the Department of Physics, Computational Condensed Matter Physics Laboratory at Jahangirnagar University,Savar, Dhaka, Bangladesh, for their software support. We would like to thank Jashore University of Science and Technology (JUST) for enabling us to execute the optimized nanostructures at the Department of Physics under the Faculty of Science.
Author information
Authors and Affiliations
Contributions
Antu Das: conceptualization, formal analysis, investigation, writing—original draft,Palash Dhali: writing, formal analysis, review and editing, Adita Afrin Oishi: writing, formal analysis, review and editing, Debashis Roy: review and editing, formal analysis, Ali Shokuhi Rad:review, Md. Mehade Hasan: visualization, writing—review and editing, supervision.
Corresponding author
Ethics declarations
Competing interests
The authors declare no competing interests.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Springer Nature or its licensor (e.g. a society or other partner) holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Das, A., Dhali, P., Oishi, A.A. et al. Adsorption behavior of hydrogen selenide gas on the surfaces of pristine and Ni-doped X12Y12 (X=Al, B and Y=N, P) nano-cages: a first-principles study. Struct Chem 34, 1439–1456 (2023). https://doi.org/10.1007/s11224-022-02105-9
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11224-022-02105-9