Abstract
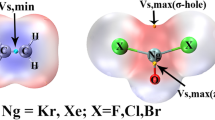
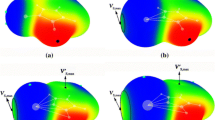
The bimolecular and termolecular complexes involving F2TO (T = Si, Ge, Sn) and XCN/BrY (X = H, Br, CH3, and PH2; Y = F, CN, OH, and H) were designed to form the π-hole tetrel bonds and different sorts of σ-hole interactions, to investigate the influence of π-hole tetrel bonds on the σ-hole interactions. The effect of π-hole tetrel bonds on the σ-hole interactions in three series HCN···F2TO···HCN, HCN···F2SiO···XCN, and HCN···F2SiO···BrY is reflected by the changes in geometry, interaction energy, and charge transfer. With the formation of π-hole tetrel bond, the VS, min value outside the oxygen atom of F2TO becomes more negative, resulting in a stronger σ-hole interaction. Comparing with the bimolecular complex, the σ-hole binding distance and binding angle in the corresponding termolecular complex changes a lot, with the formation of another tetrel bond. The σ-hole interaction energy is enhanced more than 100% in most of the complexes with the exception of HCN···F2SiO···BrCN. The enhancing effect is related to the strength of π-hole tetrel bond, but has no relationship with the strength of σ-hole interactions. In particular, the σ-hole tetrel bond between F2SiO and CH3CN varies from a weak tetrel bond in the bimolecular complex F2SiO···CH3CN to a moderate hydrogen bond in the termolecular complex HCN···F2SiO···CH3CN.






Similar content being viewed by others
References
Hobza P, Müller-Dethlefs K (2009) Chapter 1: Non-covalent interactions: theory and experiment. RSC Theoretical and Computational Chemistry Series No. 2. Royal Society of Chemistry, London
Stone AJ (2013) The theory of intermolecular forces. Oxford University Press, United Kingdom
Gilli G, Gilli P (2009) The nature of the hydrogen bond. Oxford University Press, Oxford, p 313
Metrangolo P, Neukirch H, Pilati T, Resnati G (2005). Acc Chem Res 38:386–395
Clark T, Hennemann M, Murray JS, Politzer P (2007). J Mol Model 13:291–296
Politzer P, Lane P, Concha MC, Ma YG, Murray JS (2007). J Mol Model 13:305–311
Murray JS, Lane P, Politzer P (2009). J Mol Model 15:723–729
Politzer P, Murray JS, Clark T (2010). Phys Chem Chem Phys 12:7748–7757
Murray JS, Lane P, Clark T, Riley KE, Politzer P (2012). J Mol Model 18:541–548
Politzer P, Murray JS, Clark T (2013). Phys Chem Chem Phys 15:11178–11189
Politzer P, Murray JS (2017). J Comput Chem 39:464–471
Wang WZ, Ji BM, Zhang Y (2009). J Phys Chem A 113:8132–8135
Azofra LM, Alkorta I, Scheiner S (2014). Theor Chem Accounts 133:1586–1593
Pascoe DJ, Ling KB, Cockroft SL (2017). J Am Chem Soc 139:15160–15167
Zahn S, Frank R, Hey-Hawkins E, Kirchner B (2011). Chem Eur J 17:6034–6038
Scheiner S (2013). Acc Chem Res 46:280–288
Bauzá A, Ramis R, Frontera A (2014). J Phys Chem A 118:2827–2834
Bauzá A, Mooibroek TJ, Frontera A (2015). Chem Commun 51:1491–1493
Bauzá A, Mooibroek TJ, Frontera A (2013). Angew Chem Int Ed 52:12317–12321
Bauzá A, Mooibroek TJ, Frontera A (2016). Chem Rec 16:473–487
Scheiner S (2017). J Phys Chem A 121:5561–5568
Shen SJ, Zeng YL, Li XY, Meng LP, Zhang XY (2017). Int J Quantum Chem 118:e25521–e25532
Grabowski SJ (2015). ChemPhysChem 16:1470–1479
Gao L, Zeng YL, Zhang XY, Meng LP (2016). J Comput Chem 37:1321–1327
Grabowski SJ (2018). J Comput Chem 39:472–480
Bauzá A, Frontera A (2015). Angew Chem Int Ed 54:7340–7343
Bauzá A, Frontera A (2015). ChemPhysChem 16:3625–3630
Frontera A, Bauzá A (2017). Phys Chem Chem Phys 19:30063–30068
Clark T, Murray JS, Politzer P (2018). Phys Chem Chem Phys 20:30076–30082
Clark T, Hesselmann A (2018). Phys Chem Chem Phys 20:22849–22855
Bauzá A, Mooibroek TJ, Frontera A (2015). ChemPhysChem 16:2496–2517
Bauzá A, Frontera A (2015). Chem Phys Chem 16:3108–3113
Wang H, Wang W, Jin W (2016). Chem Rev 116:5072–5104
Lehn JM (2002). Proc Natl Acad Sci U S A 99:4763–4768
Mahadevi AS, Sastry GN (2016). Chem Rev 116:2775–2825
Grabowski SJ (2014). Phys Chem Chem Phys 16:1824–1834
Gargari MS, Stilinović V, Bauzá A, Frontera A, McArdle P, Derveer DV, Ng SW, Mahmoudi G (2015). Chem Eur J 21:17951–17958
Mahmoudi G, Bauzá A, Amini M, Molins E, Mague JT, Frontera A (2016). Dalton Trans 45:10708–10716
Marín-Luna M, Alkorta I, Elguero J (2016). J Phys Chem A 120:648–656
Gholipour A (2018). Struct Chem 29:1255–126336
McDowell SAC, Joseph JA (2014). Phys Chem Chem Phys 16:10854–10860
Esrafili MD, Nurazar R, Mohammadian-Sabet F (2015). Mol Phys 113:3703–3711
Yourdkhani S, Korona T, Hadipour NL (2015). J Comput Chem 36:2412–2428
Wei Y, Cheng J, Li W, Li Q (2017). RSC Adv 7:46321–46328
Xu H, Cheng J, Yang X, Liu Z, Bo X, Li Q (2017). RSC Adv 7:21713–21720
Xu HL, Cheng JB, Yang X, Liu ZB, Li WZ, Li QM (2017). ChemPhysChem 18:2442–2450
Li W, Zeng Y, Li X, Sun Z, Meng L (2016). Phys Chem Chem Phys 18:24672–24680
Tang Q, Li Q (2014). Comput Theor Chem 1050:51–57
Guo X, Liu YW, Li QZ, Li WZ, Cheng JB (2015). Chem Phys Lett 620:7–12
Vatanparast M, Parvini E, Bahadori A (2016). Mol Phys 114:1478–1484
Frisch M, Trucks G, Schlegel HB, Scuseria G, Robb M, Cheeseman J, Scalmani G, Barone V, Mennucci B, Petersson G (2009) Gaussian 09, revision A. 02. Gaussian, Wallingford
Boys SF, Bernardi FD (1970). Mol Phys 19:553–566
Bulat FA, Toro-Labbe A, Brinck T, Murray JS, Politzer P (2010). J Mol Model 16:1679–1691
Bader RFW (1991). Chem Rev 91:893–928
Becke A, Matta CF, Boyd RJ (2007) The quantum theory of atoms in molecules. Wiley, New York
Biegler-Kôning FJ, Derdau R, Bayles D, Bader RFW (2002) AIM2000, version 2.0. University of Applied Science, Bielefeld
Johnson ER, Keinan S, Mori-Sanchez P, Contreras-Garcia J, Cohen AJ, Yang W (2010). J Am Chem Soc 132:6498–6506
Contreras-Garcia J, Johnson ER, Keinan S, Chaudret R, Piquemal JP, Beratan DN, Yang W (2011). J Chem Theory Comput 7:625–632
Lu T, Chen F (2012). J Comput Chem 33:580–592
Humphrey W, Dalke A, Schulten K (1996). J Mol Graph 14:33–38
Weinhold F, Landis C (2005) Valency and bonding, a natural bond orbital donor—acceptor perspective. Cambridge University Press, Cambridge
Su P, Li H (2009). J Chem Phys 131:014102
Michael WS, Kim KB, Jerry AB et al (1993). J Comput Chem 14:1347–1363
Frontera A, Gamez P, Mascal M, Mooibroek TJ, Reedijk J (2011). Angew Chem Int Ed 50:9564–9583
Frontera A, Gamez P, Mascal M, Mooibroek TJ, Reedijk J (2011). Angew Chem 123:9736–9756
Zhang XY, Zeng YL, Li XY, Meng LP, Zheng SJ (2011). Struct Chem 22:567–576
Li W, Zeng YL, Li XY, Sun Z, Meng LP (2015). J Comput Chem 36:1349–1358
Grabowski SJ (2017). Crystals 7:43–56
Funding
This work was supported by the Natural Science Foundation of Hebei Province (Contract Nos. B2018205198 and B2016205042), the Education Department Foundation of Hebei Province (Contract No. ZD2018066), and the Foundation of Hebei Normal University (Contract No. L2018Z04).
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
The authors declare that they have no conflict of interest.
Additional information
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
About this article
Cite this article
Wang, L., Li, X., Zeng, Y. et al. Enhancing effects of π-hole tetrel bonds on the σ-hole interactions in complexes involving F2TO (T = Si, Ge, Sn). Struct Chem 30, 1301–1313 (2019). https://doi.org/10.1007/s11224-018-1274-2
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11224-018-1274-2