Abstract
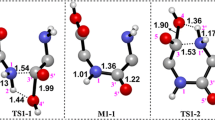
The gas-phase cyclization mechanism of cis DAA-DAA dipeptide (where DAA stands for the earlier described double amino acid molecule of (NH2)2C(COOH)2 formula while DAA-DAA indicates the system formed by two DAAs linked via the peptide bond) is investigated in the absence of any catalysts. Two different paths, concerted and stepwise, each leading to the same cyclo(DAA-DAA) dipeptide product are examined on the basis of theoretical calculations carried out at the CCSD(T)/aug-cc-pVDZ//MP2/aug-cc-pVDZ level. The final product of the cyclization was found to adopt boat conformation of the six-membered 2,5-diketopiperazine ring and its formation was predicted to be thermodynamically favored by ca. 3.7 kcal/mol. The activation barrier estimated for the concerted mechanism (39 kcal/mol) was found to be higher than each of two barriers (30–33 kcal/mol) on the stepwise route which indicates that the cyclization process leading to the cyclo(DAA-DAA) dipeptide formation is more plausible when operating along the stepwise pathway.





Similar content being viewed by others
References
Craik DJ, Fairlie DP, Liras S, Price D (2013). Chem Biol Drug Des 81:136–147
Driggers EM, Hale SP, Lee J, Terrett NK (2008). Nat Rev Drug Discov 7:608–624
Prasad C (1995). Peptides 16:151–164
McCleland K, Milne PJ, Lucieto FR, Frost C, Brauns SC, Venter MVD, Plessis JD, Dyason K (2004). J Pharm Pharmacol 56:1143–1153
Song MK, Hwang IK, Rosenthal MJ, Harris DM, Yamaguchi DT, Yip I, Go VLW (2003). Exp Biol Med 228:1338–1345
Kanoh K, Kohno S, Katada J, Takahashi J, Uno I (1999). J Antibiot 52:134–141
Nicholson B, Lloyd GK, Miller BR, Palladino MA, Kiso Y, Hayashi Y, Neuteboom STC (2006). Anti-Cancer Drugs 17:25–31
van der Merwe E, Huang D, Peterson D, Kilian G, Milne PJ, Venter MVD, Frost C (2008). Peptides 29:1305–1311
Houston DR, Synstad B, Eijsink VGH, Stark MJR, Eggleston IM, van Aalten DMF (2004). J Med Chem 47:5713–5720
Abraham WR (2005). Drug Des Rev 2:13–33
Gaunitz F, Hipkiss AR (2012). Amino Acids 43:135–142
Sinha S, Srivastava R, Clercq ED, Singh RK (2004). Nucleosides Nucleotides Nucleic Acids 23:1815–1824
Kwak MK, Liu R, Kwon JO, Kim MK, Kim AH, Kang SO (2013). J Microbiol 51:836–843
Unal CB, Owen MD, Millington WR (1997). Brain Res 747:52–59
Degeilh R, Marsh RE (1959). Acta Cryst 12:1007–1004
Fava GG, Belicchi M (1981). Acta Cryst B37:625–629
Palmer RA, Potter BS, Mendham AP, Dines TJ, Chowdhry BZ (2010). J Chem Crystallogr 40:608–615
Budesinsky M, Cisarova I, Podlaha J, Borremans F, Martins JC, Waroquierd M, Pauwelsd E (2010). Acta Cryst B66:662–677
Davies DB, Khaled Md A (1976). J Chem Soc Perkins Trans 11:1238–1244
Kopple KD, Narutis V (1981). Int J Pept Prot Res 18:33–40
Hirst JD, Persson BJ (1998). J Phys Chem A 102:7519–7524
Zhu YY, Tang MS, Shi XY, Zhao YF (2007). Int J Quantum Chem 107:745–753
Abiram A, Kolandaivel P (2010). J Mol Model 16:193–202
Wickrama Arachchilage AP, Wang F, Feyer V, Plekan O, Prince KC (2010). J Chem Phys 133:174319
Wickrama Arachchilage AP, Wang F, Feyer V, Plekan O (2012). Prince KC J Chem Phys 136:124301
Xia P, Wang C, Qi C (2013) Chinese. J Chem 31:813–818
Li Y, Li F, Zhu Y, Li X, Zhou Z, Liu C, Zhang W, Tang M (2016). Struct Chem 27:1165–1173
Freza S, Marchaj M, Skurski P (2014). Chem Phys Lett 599:34–37
Freza S (2016). Theor Chem Accounts 135:146
Freza S (2017). Theor Chem Accounts 136:7
Czapla M, Freza S (2017). Int J Quantum Chem 117:e25435
Head-Gordon M, Pople JA, Frisch MJ (1988). Chem Phys Lett 153:503–506
Frisch MJ, Head-Gordon M, Pople JA (1990). Chem Phys Lett 166:275–280
Kendall RA, Dunning Jr TH, Harrison RJ (1992). J Chem Phys 96:6796–6806
Fukui K (1981). Acc Chem Res 14:363–368
Purvis III GD, Bartlett RJ (1982). J Chem Phys 76:1910–1918
Pople JA, Head-Gordon M, Raghavachari K (1987). J Chem Phys 87:5968–5975
Lowry TH, Richardson KS (1981) Mechanism and theory in organic chemistry. 2nd edn. Harper & Row, New York p 194
Gaussian 09, Revision E.01, Frisch MJ, Trucks GW, Schlegel HB, Scuseria GE, Robb MA, Cheeseman JR, Scalmani G, Barone V, Mennucci B, Petersson GA, Nakatsuji H, Caricato M, Li X, Hratchian HP, Izmaylov AF, Bloino J, Zheng G, Sonnenberg JL, Hada M, Ehara M, Toyota K, Fukuda R, Hasegawa J, Ishida M, Nakajima T, Honda Y, Kitao O, Nakai H, Vreven T, Montgomery JA, Jr, Peralta JE, Ogliaro F, Bearpark M, Heyd JJ, Brothers E, Kudin KN, Staroverov VN, Kobayashi R, Normand J, Raghavachari K, Rendell A, Burant JC, Iyengar SS, Tomasi J, Cossi M, Rega N, Millam JM, Klene M, Knox JE, Cross JB, Bakken V, Adamo C, Jaramillo J, Gomperts R, Stratmann RE, Yazyev O, Austin AJ, Cammi R, Pomelli C, Ochterski JW, Martin RL, Morokuma K, Zakrzewski VG, Voth GA, Salvador P, Dannenberg JJ, Dapprich S, Daniels AD, Farkas Ö, Foresman JB, Ortiz JV, Cioslowski J, Fox D (2009) J Gaussian, Inc., Wallingford CT
Wright LR, Borkman RF (1982). J Phys Chem 86:3956–3962
van Dornshuld E, Vergenz RA, Tschumper GS (2014). J Phys Chem B 118:8583–8590
Ramachandran GN, Sasisekharan V (1968). Adv Prot Chem 23:283–437
Bettens FL, Bettens RPA, Brown RD, Godfrey PD (2000). J Am Chem Soc 122:5856–5860
Funding
This research was supported by the Polish Ministry of Science and Higher Education Grants No. 538-8375-B370-16/17 and DS-530-8375-D499-17. The calculations have been carried out using resources provided by Wroclaw Centre for Networking and Supercomputing (http://wcss.pl) grants No. 436.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
The author declares that she has no conflict of interest.
Ethical statement
All ethical guidelines have been adhered.
Rights and permissions
About this article
Cite this article
Freza, S. The cyclization mechanism of cis DAA-DAA dipeptide: an ab initio study. Struct Chem 29, 1025–1029 (2018). https://doi.org/10.1007/s11224-018-1085-5
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11224-018-1085-5