Abstract
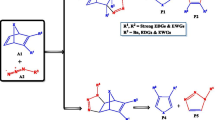
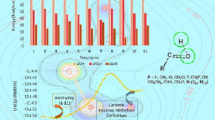
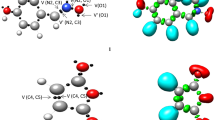
A series of neutral and protonated five-membered ring cyclic ketene acetals have been examined computationally for any trends in nucleophilicity in the exocyclic methylene and for their ground state geometries. A total of 58 different species were examined, 29 neutral molecules and the corresponding 29 protonated species. The heteroatoms that were used in the heterocyclic ring were a combination of nitrogen, phosphorus, and arsenic from the pnictogen family and oxygen, sulfur, and selenium from the chalcogen family. All geometries were initially optimized at using density functional theory and all stationary points were confirmed to be either minima or transition states through vibrational analysis. All the geometries were consequentially optimized using Møller–Plesset second order perturbation theory with a polarized triple zeta basis set. The main focus of the study was the nucleophilicity of the exocyclic methylene carbon atom and its dependence on heteroatom substitution. As probes for nucleophilicity, the proton affinities of the neutral species, the bond lengths of the exocyclic double bond, and atomic charges were used. The study also resulted in some interesting molecular geometries.


Similar content being viewed by others
References
Ye G, Chen C, Chatterjee S, Collier WE, Zhou A, Song Y, Beard DJ, Pittman C U Jr (2010) Synthesis 1:141–152
Song Y, Henry WP, Silva HI, Ye G, Pittman C U Jr (2011) Tetrahedron Lett 52:853–858
Ye G, Zhou A, Henry WP, Song Y, Chatterjee S, Beard DJ, Pittman CU Jr (2008) J Org Chem 73:5170–5172
Chatterjee S, Ye G, Song Y, Barker BL, Pittman CU Jr (2010) Synthesis 19:3384–3394
Ye G, Chatterjee S, Li M, Zhou A, Song Y, Barker BL, Chen C-L, Beard DJ, Henry WP, Pittman CU Jr (2010) Tetrahedron 66:2219–2927
Ye G, Henry WP, Chen C-L, Zhou A, Pittman CU Jr (2009) Tetrahedron Lett 50:2135–2139
Chatterjee S, Zhou A, Barker BL, Chen C-L, Song Y, Pittman CU Jr (2010) Synthesis 7:1209–1216
Zhou A, Pittman CU Jr (2006) Comb Chem 8(2):262–267
Zhou A, Pittman CU Jr (2006) Synthesis 1:37–48
Chatterjee S, Ye G, Pittman CU Jr (2010) Tetrahedron Lett 51:1139–1144
Cao L, Wu Z, Pittman CU Jr (1999) J Polym Sci A 37:2841–2852
Cao L, Pittman CU Jr (1999) J Polym Sci A 37:2823–2840
Wu Z, Cao L, Pittman CU Jr (1998) Recent Res Dev Polym Sci 2:467–484
Wu Z, Cao L, Pittman CU Jr. (1998) J Polym Sci 36:861–871 and 973–881
Zhu PC, Pittman CU Jr (1996) J Polym Sci A 34:169–174
Pittman CU Jr, Wu Z, Zhu PC (1997) J Polym Sci A 35:485–491
Zhu PC, Pittman CU Jr (1996) J Polym Sci A 34:73–80
Liu Y, Pittman CU Jr (1997) J Polym Sci A 35:3655–3771
Liu Y, Keller C, Pittman CU Jr (1997) J Polym Sci A 35:3707–3716
Zhou A, Cao L, Li H, Lu Z, Cho HS, Henry WP, Pittman CU Jr (2006) Tetrahedron 62:4093–4106
Zhu PC, Liu Y, Lin J, Pittman CU Jr (1996) J Polym Sci A 34:2195–2203
Zhou A, Pittman CU Jr (2005) Tetrahedron Lett 46(22):3801–3805
Meerwein H, Hinz G, Hoffman D, Konig E, Pfeil EJ (1939) Prakt Chem 154:83
Meerwein H (1955) Angew Chem 67:374
Meerwein H, Wunderlich K (1957) Angew Chem 69:481
Meerwein H, Allendorfer H, Beekmann P, Kunert F, Morschel H, Pawellek H, Wunderlich K (1958) Angew Chem 70(211):630
Meerwein H, Hederich V, Wunderlich K (1958) Arch Pharm 291:541
Meerwein H, Hederich V, Morschel J, Wunerlich K, Liebigs J (1960) Ann Chem 635:1
Meerwein H, Bodenbrenner K, Borner P, Kunert F, Wunderlich K, Liebigs J (1968) Ann Chem 632:38
Winstein S, Buckles REJ (1942) Am. Chem Soc 64(2780):2787
Winstein S, Hess HV, Buckles RE (1942) J Am Chem Soc 64:2769
Winstein S, Buckles RE (1943) J Am Chem Soc 65:613
Winstein S, Seymour D (1946) J Am Chem Soc 68:119
Winstein S, Grunwald E, Ingraham LL (1948) J Am Chem Soc 70:821
Winstein S, Hanson C, Grunwald E (1948) J Am Chem Soc 70:812
Winstein S, Grunwald E, Buckles RE, Hanson C (1948) J Am Chem Soc 70:816
Lemieux RU, Brice C, Huber G (1955) Can J Chem 33:134
Lemieux RU, Huber G (1955) Can J Chem 33:128
Capon B (1967) Chem Commun 21:188
Hedgley EJ, Fletcher HG Jr (1963) J Am Chem Soc 85:1615
Hedgley EJ, Fletcher HG Jr (1964) J Am Chem Soc 86(1576):1583
Pederson C (1963) Acta Chem Scand 17:1269
Pederson C (1968) Acta Chem Scand 22:1888
Hanessian S (1966) Carbohydr Res 2:86
Hanessian S, Plessas NR (1969) J Org Chem 34(1035):1045–1053
Hart H, Tomalia DA (1967) Tetrahedron Lett 8:1347
Tomalia DA, Hart H (1966) Tetrahedron Lett 7:3383–3389
Pittman CU Jr, McManus SP, Larsen JW (1972) Chem Rev 72(4):357–438
Beard DJ, Pace CR, Pittman CU Jr, Saebo S (2009) Struct Chem 20:961–967
Beard DJ, Barakat SA, Lockhart NB, Pace CR, Pittman CU Jr, Hamil BW, Saebo S (2012) Struct Chem 23:351–357
Lee C, Yang W, Parr RG (1988) Phys Rev B 37:785
Becke AD (1993) J Chem Phys 98:5648
Frisch MJ, Pople JA (1984) J Chem Phys 80:3265
Møller C, Plesset MS (1934) Phys Rev 46:618–622
Kendall RA, Dunning TH Jr, Harrison RJ (1992) J Chem Phys 96:6769
Woon DE, Dunning TH Jr (1993) J Chem Phys 98:1358
Wilson AK, Woon DE, Peterson KA, Dunning TH Jr (1999) J Chem Phys 110:7667
Baker J, Wolinski K, Malagoli M, Kinghorn D, Wolinski P, Magyarfalvi G, Saebo S, Janowski T, Pulay P (2009) J Comput Chem 30:317
PQS version 3.3, Parallel Quantum Solutions, 2013 Green Acres Road, Fayetteville, AR 72703, USA
Haddon RC (1986) Pure Appl Chem 58:137–142
Haddon RC (1988) Acc Chem Res 21:243–249
Ye G (2008) PhD Dissertation, Mississippi State University
Mulliken RS (1955) J Chem Phys 23:1833–1840
Acknowledgments
Funding for this project was provided by NSF EPSCoR #0903787. The authors are also indebted to Dr. Charles Pittman at Mississippi State University for inspiring this study and helpful discussions.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Catoire, A.E., Beard, D.J. & Saebo, S. Theoretical study of geometry and nucleophilicity of the exocyclic methylene in five-membered ring cyclic ketene acetals, neutral and protonated, containing pnictogen and chalcogen heteroatoms. Struct Chem 25, 371–376 (2014). https://doi.org/10.1007/s11224-013-0338-6
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11224-013-0338-6