Abstract
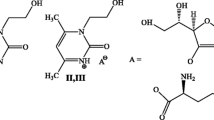
A salt-like conjugate of xymedone with para-aminobenzoic acid in a series of pyrimidine derivatives was synthesized, and its hepatoprotective properties were studied. The compound studied exhibits a cytoprotective effect at a concentration of 25 μmol L-1 enhancing the viability of normal human hepatocyte cells of the Chang Liver line by 2.1 times against the background of the impact of the d-galactosamine toxicant, which was shown by experiments in vitro. The cytotoxicity of the compound (IC50) is 20.7 mmol L-1. The data indicating the hepatoprotective effect most pronounced at the early stages of therapy were obtained in experiments in vivo performed according to the therapeutic scheme on the model of CCl4-induced toxic hepatitis. The ability of the conjugate to exert reparative and protective effects was found, since the surface area of destructive-degenerative and necrotic injuries revealed in hematoxylin- and eosin-stained sections on the third day of intraperitoneal administration of the studied compound in a dose of 0.7 mg kg-1 decreased by 1.5 times. The areas of detection of lipid inclusions in frozen sections stained with Sudan black decreased by four times on the third day at a dose of 1.7 mg kg-1, whereas the decrease was 3.2 times on the seventh day at a dose of 0.7 mg kg-1 compared to the control group of animals administrated with saline. According to the biochemical parameters, positive effects on the secretory and synthetic functions of the liver, bilirubin metabolism, and metabolism of iron and magnesium were observed during the treatment of animals with the conjugate.
Similar content being viewed by others
References
S. Li, H. Y. Tan, N. Wang, Z. J. Zhang, L. Lao, C. W. Wong, Int. J. Mol. Sci., 2015, 16, 26087.
S. G. Ismailov, V. V. Parshikov, Nizhegorodskii Med. Zh. [Nizhny Novgorod Med. J.], 2002, 3, 81 (in Russian).
V. Kh. Fazylov, I. E. Kravchenko, A. I. Fazulzyanova, F. S. Gilmullina, Metodicheskie rekomendatsii dlay vrachei (nauch-nyi obzor klinicheskikh issledovanii) [Methodical Recom men-dations for Doctors (Scientific Review of Clinical Studies)], Izd. Kazan State Medical University, Kazan, 2010, 40 pp. (in Russian).
A. B. Vyshtakalyuk, V. E. Semenov, I. A. Sudakov, K. N. Bushmeleva, L. F. Gumarova, A. A. Parfenov, N. G. Nazarov, I. V. Galyametdinova, V. V. Zobov, Russ. Chem. Bull., 2018, 67, 705.
A. B. Vyshtakalyuk, V. E. Semenov, V. V. Zobov, I. V. Galyametdinova, L. F. Gumarova, A. A. Parfenov, N. G. Nazarov, O. A. Lenina, S. A. Kondrashova, Sh. K. Latypov, G. V. Cherepnev, M. S. Shashin, V. S. Reznik, Russ. J. Bioorg. Chem., 2017, 43, 604.
A. Vyshtakalyuk, A. Parfenov, L. Gumarova, N. Nazarov, V. Zobov, I. Galyametdinova, V. Semenov, BioNanoScience, 2017, 7, 616.
A. Vyshtakalyuk, A. Parfenov, N. Nazarov, L. Gumarova, G. V. Cherepnev, I. V. Galyametdinova, V. V. Zobov, V. E. Semenov, BioNanoScience, 2018, 8, 845.
T. V. Povysheva, V. E. Semenov, I. V. Galyametdinova, V. S. Reznik, K. S. Knni, P. E. Kolesnikov, Y. A. Chelyshev, Bull. Exp. Biol. Med. (Engl. Transl.), 2016, 162, No. 8, 220.
N. J. Babu, A. Nangia, Cryst. Growth. Des., 2011, 11, 2662.
Z. Li, A. J. Matzger, Mol. Pharm., 2016, 13, 990.
R. Thakuria, B. Sarma, Crystals, 2018, 8, 101.
K. V. Drozd, A. N. Manin, A. V. Churakov, G. L. Perlovich, CrystEngComm, 2017, 19, 4273.
L. Roy, M. P. Lipert, N. Rodriguez-Hornedo, in Pharmaceutical Salts and Co-crystals, Eds J. Wouters, L. Quere, Royal Society of Chemistry, 2012, p. 247.
Ya. A. Ivanenkov, S. Yu. Maklakova, E. K. Beloglazkina, N. V. Zyk, A. G. Nazarenko, A. G. Tonevitsky, V. E. Kotelianski, A. G. Majouga, Russ. Chem. Rev., 2017, 86, 750.
N. N. Drozd, V. A. Makarov, N. T. Miftakhova, S. A. Kalugin, O. G. Stroeva, S. I. Akberova, Russ. J. Development. Biol., 2000, 31, 217.
T. P. Galbinur, E. A. Novikova, Oftalmologiya [Ophthalmology], 2012, 9, 57 (in Russian).
A. L. Kiselev, G. M. Vorobev, Vestn. Ros. Gos. Zaoch. Agrar. Univ. [Bull. of Russian Correspondence Agrarian University], 2006, 1, 129 (in Russian).
R. Mugunthu, A. Jill, C. Bykowski, G. Jeffrey, H. Ando, W. Paul, J. Chromatogr. B, 2008, 867, 247.
T. V. Povysheva, V. E. Semenov, I. V. Galyametdinova, V. S. Reznik, K. S. Knni, P. E. Kolesnikov, S. V. Kuznetsova, Yu. A. Chelyshev, Eksp. Klin. Farmakol. [Exp. Clin. Pharmacol.], 2016, 79, No. 8, 3 (in Russian).
V. S. Reznik, N. G. Pashkurov, Bull. Acad. Sci. USSR, Div. Shem. Sci., 1966, 15, 1554.
R. I. Freshney, Culture of Animal Cells: A Manual of Basic Technique and Specialized Applications, 7th ed., Wiley-Blackwell, 2016, 728 pp.
Rukovodstvo po provedeniyu doklinicheskikh issledovanii lekar-stvennykh sredstv. Ch. I [Guide for Pre-Clinical Studies of Pharmaceuticals. Part I], Ed. A. N. Mironov, Grifi K, Moscow, 2012, 944 pp. (in Russian).
National Research Council et al. Guide for the Care and Use of Laboratory Animals, National Academies Press, 2010, 246 pp.
EC Committee Guidance on Management and Care of Animals Used for Experiments and Other Research Purposes, 2007/526/ EC, of June 18th 2007.
E. S. Severin, T. L. Aleinikova, L. V. Avdeeva, L. E. Andria-nova, N. N. Belushkina, N. P. Volkova, S. A. Vorobeva, V. A. Golenchenko, A. E. Gubareva, O. V. Korlyakova, N. V. Likhacheva, N. A. Pavlova, G. V. Rubtsova, S. A. Silaeva, S. N. Siluyanova, T. A. Titova, Biokhimiya: Uchebnik [Biochemistry: Textbook], Ed. E. S. Severin, 2nd ed., GEOTARMED, Moscow, 2004, p. 472 (in Russian).
G. R. Kolokolov, E. V. Gerasina, O. L. Ananev, S. Yu. Shashlov, V. N. Shilov, O. V. Ananeva, A. Yu. Polyanina, Analizy. Polnyi spravochnik [Full Reference Book], Eksmo, Moscow, 2008, p. 218 (in Russian).
Author information
Authors and Affiliations
Corresponding authors
Additional information
Based on the materials of the IV Interdisciplinary Symposium on Medical, Organic, Biological Chemistry and Pharmaceutics (MOBI-ChemPharma 2018) (September 23–26, 2018, Novyi Svet, Crimea).
Published in Russian in Izvestiya Akademii Nauk. Seriya Khimicheskaya, No. 12, pp. 2307–2315, December, 2019.
Rights and permissions
About this article
Cite this article
Parfenov, A.A., Vyshtakalyuk, A.B., Gumarova, L.F. et al. Xymedone conjugate with para-aminobenzoic acid. Estimation of hepatoprotective properties. Russ Chem Bull 68, 2307–2315 (2019). https://doi.org/10.1007/s11172-019-2704-z
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11172-019-2704-z