Abstract
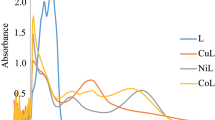
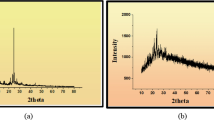
Three Schiff base ligands [H2L1–H2L3] containing nitrogen/oxygen donor atoms and their Co(II), Ni(II), Cu(II) and Zn(II) complexes were synthesized by stirring metal acetates with Schiff base ligands obtained from condensation reaction of 2-amino-6-chloro-4-nitrophenol with 5-chloro salicylaldehyde/3,5-dibromo salicylaldehyde/3-methoxy-5–nitro salicylaldehyde. The structural traits of the synthesized compounds were done by using elemental analysis, spectroscopic techniques (UV–Vis, 1H and 13C NMR, FT-IR), mass spectrometry and some physical studies (XRD, TGA). According to spectral data, ligands behave as a tridentate (ONO) and formed complexes with octahedral geometry. The thermogravimetric analysis revealed that metal complexes decay in multi-steps leaving metal oxide as an end product. Powder XRD study suggested crystalline nature of the compounds. The energy gap (HOMO–LUMO) and molecular electrostatic potential calculation were computed by using DFT/B3LYP/6-31G** basis set. Derived ligands and complexes were explored for in vitro antimicrobial potential toward two gram-positive bacteria, two gram-negative bacteria, i.e., S. aureus, B. subtilis, P. aeruginosa, E. coli, and two fungal strains, i.e., A. niger, C. albicans, through serial dilution method taking ciprofloxacin and fluconazole as standard. The investigated results showed that complexes are more potent than free Schiff base ligands. The Cu(L2)(H2O)3 (0.0115 μmol/mL) and Zn(L2)(H2O)3 (0.0115 μmol/mL) complexes were found to be more active among all the investigated compounds. Additionally, molecular docking studies were also performed for some compounds in the active site of DNA Gyrase enzyme (PDB code: 1AJ6), suggesting good hydrophobic interactions of compounds with the enzyme.










Similar content being viewed by others
Availability of data and materials
All the spectra are provided in the supplementary information.
References
I. Rama, R. Selvameena, J. Indian Chem. Soc. 97, 2144 (2020)
R.R. Gupta, M. Kumar, M.V. Gupta. J. Heterocycl. Chem. 357 (1998)
A. Xavier, N. Srividhya, N. IOSR. J. Appl. Chem. 7, 06 (2014)
M. Sani, A. Nuraddeen, Chem. Search J. 10, 54 (2019)
M. Munjal, J. pharmacogn. Phytochem. 7, 864 (2019)
A. Singh, H. Kaur, S. Arora, P.M.S. Bedi, Arch. Pharm. 355, 2100368 (2022)
S.M. El-Megharbel, N.M. Al-Baqami, E.H. Al-Thubaiti, S.H. Qahl, B. Albogami, R.Z. Hamza, Curr. Issues Mol. Biol. 44, 1810 (2022)
S.K. Tadavi, J.D. Rajput, S.D. Bagul, A.A. Hosamani, J.N. Sangshetti, R.S. Bendre, Res. Chem. Intermed. 43, 4863 (2017)
N. Raman, S. Johnson Raja, A. Sakthivel, J. Coord. Chem. 62, 691 (2017)
A. Gubendran, M.P. Kesavan, S. Ayyanaar, J.D. Raja, P. Athappan, J. Rajesh, Appl. Organomet. Chem. 31, e3708 (2017)
X. Liu, Y. Sun, M. Lu, X. Pan, Z. Wang, J. Adhes. Sci. Technol. 35, 63 (2021)
H. Kargar, A.A. Ardakani, M.N. Tahir, M. Ashfaq, K.S. Munawar, J. Mol. Struct. 1229, 129842 (2021)
A. Jaafar, N. Mansour, A. Fix-Tailler, M. Allain, W.H. Faour, W.N. Shebaby, G. Ibrahim, Chem. Sel. 7, e202104497 (2022)
S. Kumar, J. Devi, V.D. Ghule, Res. Chem. Intermed. 48, 3497 (2022)
J. Devi, S. Sharma, S. Kumar, D.K. Jindal, P.P. Dutta, D. Kumar, Res. Chem. Intermed. 47, 2433 (2021)
I.P. Ejidike, M.O. Bamigboye, H.S. Clayton, Spectrosc. Lett. 54, 212 (2021)
P.L. Hegde, S.S. Bhat, V.K. Revankar, S.A. Shaikh, K. Kumara, N.K. Lokanath, J. Mol. Struct. 1257, 132589 (2022)
V.D. Ragole, D.S. Wankhede, S.V. Gayakwad, Inorg. Nano-Met. Chem. 1234, 52 (2022)
J. Devi, S. Pachwania, J. Yadav, A. Kumar, Phosphorus Sulfur Silicon Relat. Elem. 196, 1049 (2021)
M. Yadav, S. Sharma, J. Devi, J. Chem. Sci. 133, 1 (2021)
C.A. Conde, M.V. De Almeida, G.D.S. Da Silva, M.B.P.D.A. Sodre, J.C.F. Rodrigues, M. Navarro, Transit. Metals Chem. 47, 147 (2022)
Y. Deswal, S. Asija, A. Dubey, L. Deswal, D. Kumar, D.K. Jindal, J. Devi, J. Mol. Struct. 1253, 132266 (2022)
A. Ashraf, M.G. El-Desouky, M.A. El-Afify, Biointerface. Res. Appl. Chem. 12, 1053 (2021)
I.B. Amali, M.P. Kesavan, V. Vijayakumar, N.I. Gandhi, J. Rajesh, G. Rajagopal, J. Mol. Struct. 1183, 342 (2019)
A. Zianna, G. Geromichalos, E. Psoma, S. Kalogiannis, A.G. Hatzidimitriou, G. Psomas, J. Inorg. Biochem. 229, 111727 (2022)
J. Devi, S. Kumar, B. Kumar, S. Asija, A. Kumar, Res. Chem. Intermed. 48, 1541 (2022)
A.D. Becke, J. Chem. Phys. 98, 1372 (1993)
S. Khokhar, Y. Feng, M.R. Campitelli, R.J. Quinn, J.N. Hooper, M.G. Ekins, R.A. Davis, A.-D. Trikentramides, J. Nat. Prod. 76, 2100 (2013)
C. Lee, W. Yang, R.G. Paar, J. Phys. Condens. Matter. 37, 785 (1998)
K. Muthusamy, S. Chinnasamy, S. Nagarajan, T. Sivaraman, S. Chinnasamy, J. Biomol. Struct. Dyn. 35, 1936 (2017)
W.J. Pietro, M.M. Francl, W.J. Hehre, D.J. DeFrees, J.A. Pople, J.S. Binkley, J. Am. Chem. Soc. 104, 5039 (1982)
Glide, Version 6.4, Schrodinger, LLC, New York, NY, (2018)
G.A. Holdgate, A. Tunnicliffe, W.H. Ward, S.A. Weston, G. Rosenbrock, P.T. Barth, I.W. Taylor, R.A. Pauptit, D. Timms, Biochem. 36, 9663 (1997)
P.W. Rose, A. Prlic, C. Bi, W.F. Bluhm, C.H. Christie, S. Dutta, R.K. Green, D.S. Goodsell, J.D. Westbrook, J. Woo. Nucl. Acids Res. 43, D345 (2015)
G.M. Sastry, M. Adzhigirey, T. Day, R. Annabhimoju, W. Sherman, J. Comput. Aided Mol. Des. 27, 221 (2013)
A. Dubey, A. Marabotti, P.W. Ramteke, A. Facchiano, Future. Med. Chem. 8, 841 (2016)
A. Dubey, A. Marabotti, P.W. Ramteke, A. Facchiano, Biochem. Biophys. Res. Commun. 473, 449 (2016)
S. Bharadwaj, A. Dubey, N.K. Kamboj, A.K. Sahoo, S.G. Kang, U. Yadava, Sci Rep. 11, 10169 (2021)
A. Dubey, S. Dotolo, P.W. Ramteke, A. Facchiano, A Marabotti. Biomol. 9, 5 (2018)
S.E. Abd El-Razek, S.M. El-Gamasy, M. Hassan, M.S. Abdel-Aziz, S.M. Nasr, J. Mol. Struct. 1203, 127381 (2020)
J. Devi, J. Yadav, D. Kumar, D.K. Jindal, B. Basu, Appl. Organomet. Chem. 34, e5815 (2020)
J. Devi, M. Yadav, A. Kumar, A. Kumar, Chem. Pap. 72, 2479 (2018)
N.N. Rao, K. Gopichand, R. Nagaraju, A.M. Ganai, P.V. Rao, Chem. Data Collect. 27, 100368 (2020)
L. John, R.S. Joseyphus, I.H. Joe, SN App. Sci. 2, 1 (2020)
Y. Deswal, S. Asija, D. Kumar, D.K. Jindal, G. Chandan, V. Panwar, N. Kumar, Res. Chem. Intermed. 48, 703 (2022)
A. Gulzar, T. Mahmud, L. Mitu, R. Munir, M. Imran, K. Iftikhar, Rev. De Chim. 70, 596 (2019)
P. Ghorai, R. Saha, S. Bhuiya, S. Das, P. Brandão, D. Ghosh, A. Saha, Polyhedron 141, 153 (2018)
R. Konakanchi, J. Haribabu, J. Prashanth, V.B. Nishtala, R. Mallela, S. Manchala, L.R. Kotha, Appl. Organomet. Chem. 32, e4415 (2018)
A.A.A. Aziz, A.N.M. Salem, M.A. Sayed, M.M. Aboaly, J. Mol. Struct. 1010, 130 (2012)
R. Kumar, S. Obrai, A.K. Jassal, M.S. Hundal, J. Mitra, S. Sharma, J. Coord. Chem. 68, 2130 (2015)
E. Bursal, F. Turkan, K. Buldurun, N. Turan, A. Aras, N. Çolak, M.C. Yergeri, Biometals 34, 393 (2021)
A. Palanimurugan, A. Dhanalakshmi, P. Selvapandian, A. Kulandaisamy, Heliyon. 5, e02039 (2019)
M. Azam, S.M. Wabaidur, M. Alam, Z. Khan, I.O. Alanazi, S.I. Al-Resayes, I.S. Moon, Transit. Met. Chem. 46, 65 (2021)
C. Shiju, D. Arish, S. Kumaresan, J. Mol. Struct. 1221, 128770 (2020)
L.H. Abdel-Rahman, A.M. Abu-Dief, R.M. El-Khatib, S.M. Abdel-Fatah, J. Photochem. Photobiol. B Biol. 162, 298 (2016)
J.Y. Al-Humaidi, J. Mol. Struct. 1183, 190 (2019)
J. Devi, M. Yadav, D. Kumar, L.S. Naik, D.K. Jindal, Appl. Organomet. Chem. 33, e4693 (2019)
P. Tyagi, M. Tyagi, S. Agrawal, S. Chandra, H. Ojha, M. Pathak, Spectrochim. Acta A Mol. Biomol. Spectrosc. 171, 246 (2017)
H.F. Abd El-Halim, G.G. Mohamed, M.N. Anwar, Appl. Organomet. Chem. 32, e3899 (2018)
R. Kalarani, M. Sankarganesh, G.V. Kumar, M. Kalanithi, J. Mol. Struct. 1206, 127725 (2020)
S. Bharadwaj, A. Dubey, U. Yadava, S.K. Mishra, S.G. Kang, V.D. Dwivedi, Brief. Bioinform. 22, 1361 (2021)
Acknowledgements
The author Mr. Sanjeev Kumar (SRF) (File No. 09/752(0095)/2019-EMR-I) is highly thankful to CSIR, New Delhi, India, for the grant of fellowship and thankful to Dr. A.P.J. Abdul Kalam Central Instrumentation Laboratory and Department of Chemistry, GJUS&T, Hisar, for providing good facilities to perform this research work.
Funding
Author Sanjeev Kumar (SRF) (File No. 09/752(0095)/2019-EMR-I), acknowledges the CSIR, New Delhi, India, for financial assistance in form of Senior Research Fellowship.
Author information
Authors and Affiliations
Contributions
SK helped in writing—original draft; analysis, validation; conceptualization; data curation; methodology; software; visualization. JD performed formal analysis; supervision; validation. AD contributed to writing—original draft, validation, software. DG developed software. DKJ, AS and SA helped in formal analysis.
Corresponding author
Ethics declarations
Conflict of interest
The authors declare that they have no conflict of interest.
Ethical approval
No animal/human studies were carried out in the present work.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary Information
Below is the link to the electronic supplementary material.
Rights and permissions
Springer Nature or its licensor (e.g. a society or other partner) holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Kumar, S., Devi, J., Dubey, A. et al. Co(II), Ni(II), Cu(II) and Zn(II) complexes of Schiff base ligands: synthesis, characterization, DFT, in vitro antimicrobial activity and molecular docking studies. Res Chem Intermed 49, 939–965 (2023). https://doi.org/10.1007/s11164-022-04941-0
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11164-022-04941-0