Abstract
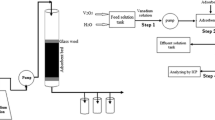
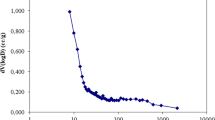
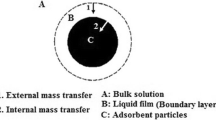
In this study, iron functional groups-impregnated activated carbon (IIAC) composite was prepared as a novel adsorbent for vanadium separation. Adsorption experiments were performed in batch and column systems, and the effects of various operating parameters, such as solution pH, initial concentration, contact time, and temperature, were evaluated. The kinetic data confirmed the validity of the pseudo-second-order kinetic model for vanadium adsorption on IIAC. The sorption equilibrium data were analyzed using Langmuir, Freundlich, and Dubinin–Radushkevich isotherm models. The results showed that IIAC has a vanadium ions adsorption capacity of 313 mg g−1. The activation and thermodynamic parameters were determined using kinetics and equilibrium data. The experimental data of the column adsorption process were fitted by Thomas and BDST models. The results showed that Thomas model can well describe the breakthrough curves. The column experiments showed that IIAC composite has good adsorption performance for vanadium ions adsorption.









Similar content being viewed by others
References
H. Wyers, Brit. J. Ind. Med. 3, 177–182 (1946)
B. Patel, G.E. Henderson, S.J. Haswell, R. Grzeskowiak, The Analyst 115, 1063–1066 (1990)
A.P. Rodríguez, J.A.H. Viezcas, J.R.P. Videa, G.L.G. Torresdey, O.P. Pérez, F.R.R. Velázquez, Microchem. J. 118, 1–11 (2015)
A. Dabrowski, Z. Hubicki, P. Podkoscielny, E. Robens, Chemosphere 56, 91–106 (2004)
A. Alibrahim, H. Shlewit, S. Alike, Chem. Eng. J. 52(1), 29–33 (2008)
M. Nabavinia, M. Soleimani, A. Kargari, Int. J. Chem. Environ. Eng. 3, 149–152 (2012)
R. Navarro, J. Guzman, I. Saucedo, J. Revilla, E. Guibal, Waste Manag. 27, 425–438 (2007)
N. Mehrabi, M. Soleimani, M.M. Yeganeh, H. Sharififard, RSC Adv. 5, 51470–51482 (2015)
R. Chand-Bansal, M. Goyal, Activated Carbon Adsorption (Taylor & Francis, Boca Raton, 2005)
H. Marsh, F. Rodriguez-Reinoso, Activated Carbon (Elsevier, New York, 2006)
A.M. Cooper, K.D. Hristovski, T. Möller, P. Westerhoff, P. Sylvester, J. Hazard. Mater. 183, 381–388 (2010)
J.A. Arcibar-Orozco, J.R. Rangel-Mendez, T.J. Bandosz, J. Hazard. Mater. 246–247, 300–309 (2013)
J.H. Xu, N. Gao, Y. Deng, S. Xia, Chem. Eng. J. 222, 520–526 (2013)
H. Sharififard, M. Soleimani, RSC Adv. 5, 80650–80660 (2015)
H. Sharififard, M. Soleimani, Res. Chem. Intermed. 43, 2501–2516 (2017)
H. Sharififard, F. Zokaee Ashtiani, M. Soleimani, Asia Pac. J. Chem. Eng. 8, 384–395 (2013)
P. Cambier, Clay Miner. 21, 191–200 (1986)
Z. Al-Qodah, R. Shawabkah, Braz. J. Chem. Eng. 26, 127–136 (2009)
Y. Li, C. Zhu, T. Lu, Z. Guo, D. Zhang, J. Ma, S. Zhu, Carbon 52, 565–573 (2013)
T.S. Anirudhan, P.G. Radhakrishnan, Chem. Eng. J. 165, 142–150 (2010)
T. Wang, Z. Cheng, B. Wang, W. Ma, Chem. Eng. J. 181–182, 182–188 (2012)
B.H. Hameed, A.A. Ahmad, J. Hazard. Mater. 164, 870–875 (2009)
M.C. Ncibi, J. Hazard. Mater. 153, 207–212 (2008)
K. Riahi, S. Chaabane, B.B. Thayer, J. Saudi Chem. Soc. 21, 143–152 (2017)
V.J. Inglezakis, A.A. Zorpas, Desalin. Water Treat. 39, 149–157 (2012)
C. Namasivayam, D. Sangeetha, Adsorption 12, 103–117 (2006)
J. Guzman, I. Saucedo, R. Navarro, J. Revilla, E. Guibal, Langmuir 18, 1567–1573 (2002)
M. Jansson-Charrier, E. Guibal, J. Roussy, B. Delanghe, P. Le Cloirec, Water Res. 30, 465–475 (2002)
T.S. Anirudhan, S. Jalajamony, L. Divya, Ind. Eng. Chem. Res. 48, 2118–2124 (2009)
X.P. Liao, W. Tang, R.Q. Zhou, B. Shi, Adsorption 14, 55–64 (2008)
T. Leiviskä, A. Keränen, N. Vainionpää, J. Al Amir, O. Hormi, J. Tanskanen, Water Sci. Technol. 72, 437–442 (2015)
A. Naeem, P. Westerhoff, S. Mustafa, Water Res. 41, 1596–1602 (2007)
M. Govindaraj, S. Pattabhi, Desalin. Water Treat. 54, 2664–2674 (2015)
A. Bhatnagar, A.K. Minocha, D. Pudasainee, H.K. Chung, S.H. Kim, H.S. Kim, G. Lee, B. Min, B.H. Jeon, Chem. Eng. J. 144, 197–204 (2008)
D.M. Manohar, B.F. Noeline, T.S. Anirudhan, Ind. Eng. Chem. Res. 44, 6676–6684 (2005)
C. Pennesi, C. Totti, F. Beolchini, PLoS ONE 8(10), e76870 (2013)
A. Keränen, T. Leiviskä, A. Salakka, J. Tanskanen, Desalin. Water Treat. 53, 2645–2654 (2015)
Y. Shi, J. Yang, W. Mao, Y. Li, X. Xu, H. Zhang, W. Yu, Y. Li, C. Yang, Desalin. Water Treat. 53, 2655–2663 (2015)
R.G. Kunz, J.F. Giannelli, H.D. Stensel, J. Water Pollut. Control Feder. 48, 762–770 (1976)
M. Songolzadeh, M. Soleimani, M. Takht Ravanchi, J. Nat. Gas Sci. Eng. 27, 831–841 (2015)
R. Han, Y. Wang, W. Yu, W. Zou, J. Shi, H. Lui, J. Hazard. Mater. 139, 513–518 (2006)
M. Ghasemi, A.R. Keshtkar, R. Dabbagh, S. Jaber Safdari, J. Hazard. Mater. 189(1–2), 141–149 (2011)
Z. Xu, J.G. Cai, B.C. Pan, J. Zhejiang Univ. Sci. A 14, 155–176 (2013)
G.S. Bohart, E.Q. Adams, J. Chem. Soc. 42, 523–529 (1920)
A.B. Albadarin, C. Mangwandi, A.H. Al-Muhtaseb, G.M. Walker, S.J. Allen, M.N.M. Ahmad, Chin. J. Chem. Eng. 20(3), 469–477 (2012)
Acknowledgement
The authors wish to acknowledge the Ministry for Foreign Affairs and International Cooperation of Italy for Financial support.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Sharififard, H., Pepe, F., Aprea, P. et al. Chemical modification of activated carbon surface with iron functional groups for efficient separation of vanadium: batch and column study. Res Chem Intermed 43, 6553–6570 (2017). https://doi.org/10.1007/s11164-017-3004-6
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11164-017-3004-6