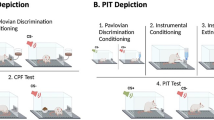
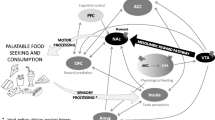
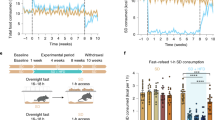
Abstract
Obesity, Type 2 diabetes and other metabolic disorders continue to pose serious challenges to human health and well-being. An important source of these challenges is the overconsumption of saturated fats and sugar, main staples of what has been called the Western-style diet (WD). The current paper describes a theoretical model and supporting evidence that links intake of a WD to interference with a specific brain substrate that underlies processing of interoceptive signals of hunger and satiety. We review findings from rats and humans that the capacity of these signals to modulate the strength of appetitive and eating behavior depends on the functional integrity of the hippocampus and the learning memory operations it performs. Important among these operations is the use of contextual information to retrieve memories that are associated with other events. Within our framework, satiety provides an interoceptive context that informs animals that food cues and appetitive behavior will not be followed by rewarding postingestive outcomes. This serves to prevent those cues and responses from retrieving those reward memories. The findings reviewed provide evidence that consuming a WD and the high amounts of saturated fat and sugar it contains (a) is associated with the emergence of pathophysiologies to which the hippocampus appears selectively vulnerable (b) impairs hippocampal-dependent learning and memory (HDLM) and (c) weakens behavioral control by interoceptive hunger and satiety contextual stimuli. It is hypothesized that these consequences of WD intake may establish the conditions for a vicious cycle of further WD intake, obesity, and potentially cognitive decline.








Similar content being viewed by others
References
Chen WG, Schloesser D, Arensdorf AM, Simmons JM, Cui C, Valentino R, et al. The emerging science of interoception: sensing, integrating, interpreting, and regulating signals within the self. Trends Neurosci. 2021;44:3–16.
Jones S, Sample CH, Hargrave SL, Davidson TL. Associative mechanisms underlying the function of satiety cues in the control of energy intake and appetitive behavior. Physiol Behav Elsevier Inc. 2018;192:37–49.
Herbert BM, Pollatos O. Attenuated interoceptive sensitivity in overweight and obese individuals. Eat Behav. 2014;15:445–8. Available from: https://pubmed-ncbi-nlm-nih-gov.proxyau.wrlc.org/25064297/.
Simmons WK, DeVille DC. Interoceptive contributions to healthy eating and obesity. Curr Opin Psychol. 2017;17:106–12. Available from: https://pubmed-ncbi-nlm-nih-gov.proxyau.wrlc.org/28950955/.
Davidson TL, Jones S, Roy M, Stevenson RJ. The cognitive control of eating and body weight: It’s more than what you think. Front Psychol. 2019;10.
Davidson TL, Sample CH, Swithers SE. An application of Pavlovian principles to the problems of obesity and cognitive decline. Neurobiol Learn Mem 2014;172–84. Available from: https://pubmed-ncbi-nlm-nih-gov.proxyau.wrlc.org/23887140/.
Davidson TL, Kanoski SE, Walls EK, Jarrard LE. Memory inhibition and energy regulation. Physiol Behav. 2005;86:731–46. Available from: https://pubmed-ncbi-nlm-nih-gov.proxyau.wrlc.org/16263144/.
Eichenbaum H. Prefrontal-hippocampal interactions in episodic memory. Nat Rev Neurosci. 2017;18:547–58. Available from: https://pubmed-ncbi-nlm-nih-gov.proxyau.wrlc.org/28655882/.
Maurer AP, Nadel L. The Continuity of Context: A Role for the Hippocampus. Trends Cogn Sci. 2021;25:187–99. Available from: https://pubmed-ncbi-nlm-nih-gov.proxyau.wrlc.org/33431287/.
Sugar J, Moser MB. Episodic memory: Neuronal codes for what, where, and when. Hippocampus. 2019;29:1190–205. Available from: https://pubmed-ncbi-nlm-nih-gov.proxyau.wrlc.org/31334573/.
Østbye T, Malhotra R, Landerman LR. Body mass trajectories through adulthood: Results from the National Longitudinal Survey of Youth 1979 cohort (1981-2006). Int J Epidemiol. 2011;40:240–50. Available from: https://pubmed-ncbi-nlm-nih-gov.proxyau.wrlc.org/20819785/.
Stevenson RJ, Francis HM. The hippocampus and the regulation of human food intake. Psychol Bull. 2017;143:1011–32.
Kopp W. How western diet and lifestyle drive the pandemic of obesity and civilization diseases. Diabetes Metab Syndr Obes Targets Ther. 2019;12:2221–36. Available from: https://pubmed-ncbi-nlm-nih-gov.proxyau.wrlc.org/31695465/.
Medina-Remón A, Kirwan R, Lamuela-Raventós RM, Estruch R. Dietary patterns and the risk of obesity, type 2 diabetes mellitus, cardiovascular diseases, asthma, and neurodegenerative diseases. Crit Rev Food Sci Nutr. 2018;58:262–96. Available from: https://pubmed-ncbi-nlm-nih-gov.proxyau.wrlc.org/27127938/.
Barrett LF, Simmons WK. Interoceptive predictions in the brain. Nat Rev Neurosci. Nature Publishing Group. 2015;419–29. Available from: https://pubmed-ncbi-nlm-nih-gov.proxyau.wrlc.org/26016744/.
Craig AD. How do you feel - now? The anterior insula and human awareness. Nat Rev Neurosci. 2009;59–70. Available from: https://pubmed-ncbi-nlm-nih-gov.proxyau.wrlc.org/19096369/.
Al-Zubaidi A, Iglesias S, Stephan KE, Buades-Rotger M, Heldmann M, Nolde JM, Effects of hunger, satiety and oral glucose on effective connectivity between hypothalamus and insular cortex. Neuroimage. 2020;217. Available from: https://doi.org/10.1016/j.neuroimage.2020.116931.
Haaranen M, Scuppa G, Tambalo S, Järvi V, Bertozzi SM, Armirotti A, et al. Anterior insula stimulation suppresses appetitive behavior while inducing forebrain activation in alcohol-preferring rats. Transl Psychiatry. 2020;10. Available from: https://pubmed-ncbi-nlm-nih-gov.proxyau.wrlc.org/32424183/.
Livneh Y, Sugden AU, Madara JC, Essner RA, Flores VI, Sugden LA, et al. Estimation of current and future physiological states in insular cortex. Neuron. 2020;105:1094-1111.e10. Available from: https://pubmed-ncbi-nlm-nih-gov.proxyau.wrlc.org/31955944/.
Hebben N, Corkin S, Eichenbaum H, Shedlack K. Diminished ability to interpret and report internal states after bilateral medial temporal resection: Case H.M. Behav Neurosci. 1985;99:1031–9. Available from: https://pubmed-ncbi-nlm-nih-gov.proxyau.wrlc.org/3843537/.
Assaf Y, Bouznach A, Zomet O, Marom A, Yovel Y. Conservation of brain connectivity and wiring across the mammalian class. Nat Neurosci. 2020;23:805–8. Available from: https://pubmed-ncbi-nlm-nih-gov.proxyau.wrlc.org/32514137/.
Bunsey M, Elchenbaum H. Conservation of hippocampal memory function in rats and humans. Nature. 1996;379:255–7. Available from: https://pubmed-ncbi-nlm-nih-gov.proxyau.wrlc.org/8538790/.
Davidson TL, Kanoski SE, Chan K, Clegg DJ, Benoit SC, Jarrard LE. Hippocampal lesions impair retention of discriminative responding based on energy state cues. Behav Neurosci. 2010;124:97–105.
Davidson TL, Monnot A, Neal AU, Martin AA, Horton JJ, Zheng W. The effects of a high-energy diet on hippocampal-dependent discrimination performance and blood-brain barrier integrity differ for diet-induced obese and diet-resistant rats. Physiol Behav. 2012;107:26–33.
Sample CH, Jones S, Hargrave SL, Jarrard LE, Davidson TL. Western diet and the weakening of the interoceptive stimulus control of appetitive behavior. Behav Brain Res. Elsevier B.V. 2016;312:219–30.
Kanoski SE, Walls EK, Davidson TL. Interoceptive satiety signals produced by leptin and CCK. Peptides. 2007;28:988–1002.
Davidson TL, Carretta JC. Cholecystokinin, but not bombesin, has interoceptive sensory consequences like 1-h food deprivation. Physiol Behav. 1993;53:737–45. Available from: https://pubmed-ncbi-nlm-nih-gov.proxyau.wrlc.org/8511180/.
Davidson TL, Kanoski SE, Tracy AL, Walls EK, Clegg D, Benoit SC. The interoceptive cue properties of ghrelin generalize to cues produced by food deprivation. Peptides. 2005;26:1602–10. Available from: https://pubmed-ncbi-nlm-nih-gov.proxyau.wrlc.org/16112399/.
Benoit SC, Davidson TL. Interoceptive sensory signals produced by 24-hr food deprivation, pharmacological glucoprivation, and lipoprivation. Behav Neurosci. 1996;110:168–80.
Davidson TL, Chan K, Jarrard LE, Kanoski SE, Clegg DJ, Benoit SC. Contributions of the hippocampus and medial prefrontal cortex to energy and body weight regulation. Hippocampus. 2009;19:235–52. Available from: https://pubmed-ncbi-nlm-nih-gov.proxyau.wrlc.org/18831000/.
Hannapel R, Ramesh J, Ross A, Lalumiere RT, Roseberry AG, Parent MB. Postmeal optogenetic inhibition of dorsal or ventral hippocampal pyramidal neurons increases future intake. eNeuro. 2019;6.
Hannapel RC, Henderson YH, Nalloor R, Vazdarjanova A, Parent MB. Ventral hippocampal neurons inhibit postprandial energy intake. Hippocampus. John Wiley and Sons Inc. 2017;27:274–84.
Henderson YO, Smith GP, Parent MB. Hippocampal neurons inhibit meal onset. Hippocampus. 2013;23:100–7. Available from: https://pubmed-ncbi-nlm-nih-gov.proxyau.wrlc.org/22927320/.
Davidson TL. The nature and function of interoceptive signals to feed: toward integration of physiological and learning perspectives. Psychol Rev. 1993;100:640–57.
Clasen MM, Riley AL, Davidson TL. Hippocampal-dependent inhibitory learning and memory processes in the control of eating and drug taking. Curr Pharm Des. 2020.
Davidson TL, Sample CH, Swithers SE. An application of Pavlovian principles to the problems of obesity and cognitive decline. Neurobiol Learn Mem. 2014;108:172–84. Available from: https://www.ncbi.nlm.nih.gov/pubmed/23887140.
Todd TP, Winterbauer NE, Bouton ME. Contextual control of appetite. Renewal of inhibited food-seeking behavior in sated rats after extinction. Appetite. 2012;58:484–9. Available from: https://pubmed.ncbi.nlm.nih.gov/22200411/.
Schepers ST, Bouton ME. Hunger as a context: food seeking that is inhibited during hunger can renew in the context of satiety. Psychol Sci. SAGE Publications Inc. 2017;28:1640–8.
Urcelay GP, Miller RR. The functions of contexts in associative learning. Behav Process. 2014;104:2–12. Available from: https://pubmed-ncbi-nlm-nih-gov.proxyau.wrlc.org/24614400/.
Bouton ME. Context and behavioral processes in extinction. Learn Mem. 2004;11:485–94.
Fraser KM, Holland PC. Occasion setting. Behav Neurosci. 2019;133:145–75.
Kennedy PJ, Shapiro ML. Motivational states activate distinct hippocampal representations to guide goal-directed behaviors. Proc Natl Acad Sci U S A. 2009;106:10805–10. Available from: https://pubmed-ncbi-nlm-nih-gov.proxyau.wrlc.org/19528659/.
Smith DM, Bulkin DA. The form and function of hippocampal context representations. Neurosci Biobehav Rev. 2014;40:52–61. Available from: https://pubmed-ncbi-nlm-nih-gov.proxyau.wrlc.org/24462752/.
Eichenbaum H, Sauvage M, Fortin N, Komorowski R, Lipton P. Towards a functional organization of episodic memory in the medial temporal lobe. Neurosci Biobehav Rev. 2012;36:1597–608. Available from: https://pubmed-ncbi-nlm-nih-gov.proxyau.wrlc.org/21810443/.
Maren S, Holt W. The hippocampus and contextual memory retrieval in Pavlovian conditioning. Behav Brain Res. 2000;110:97–108. Available from: https://pubmed-ncbi-nlm-nih-gov.proxyau.wrlc.org/10802307/.
Urcelay GP. Competition and facilitation in compound conditioning. J Exp Psychol Anim Learn Cogn Am Psychol Assoc Inc. 2017;303–14. Available from: https://pubmed-ncbi-nlm-nih-gov.proxyau.wrlc.org/28795826/.
Boddez Y, Haesen K, Baeyens F, Beckers T. Selectivity in associative learning: A cognitive stage framework for blocking and cue competition phenomena. Front Res Psychol. 2014;5. Available from: https://pubmed-ncbi-nlm-nih-gov.proxyau.wrlc.org/25429280/.
Wasserman EA, Miller RR. What’s elementary about associative learning? Annu Rev Psychol. 1997;48:573–607. Available from: https://pubmed-ncbi-nlm-nih-gov.proxyau.wrlc.org/9046569/.
Holman JG, Mackintosh NJ. The control of appetitive instrumental responding does not depend on classical conditioning to the discriminative stimulus. Q J Exp Psychol Sect B. 1981;33:21–31.
Schachter S. Obesity and eating: Internal and external cues differentially affect eating behavior of obese and normal subjects. Science (80-). 1968;161:751–6.
Tran DMD, Westbrook RF. A high-fat high-sugar diet-induced impairment in place-recognition memory is reversible and training-dependent. Appetite. 2017;110:61–71.
Kanoski SE, Davidson TL. Different Patterns of Memory Impairments Accompany Short- and Longer-Term Maintenance on a High-Energy Diet. J Exp Psychol Anim Behav Process. 2010;36:313–9. Available from: https://pubmed-ncbi-nlm-nih-gov.proxyau.wrlc.org/20384410/.
Beilharz JE, Maniam J, Morris MJ, Short-term exposure to a diet high in fat and sugar, or liquid sugar, selectively impairs hippocampal-dependent memory, with differential impacts on inflammation. Behav Brain Res. 2016;306:1–7. Available from: https://doi.org/10.1016/j.bbr.2016.03.018.
Beilharz JE, Maniam J, Morris MJ. Short exposure to a diet rich in both fat and sugar or sugar alone impairs place, but not object recognition memory in rats. Brain Behav Immun. 2014;37:134–41.
Yeomans MR. Adverse effects of consuming high fat-sugar diets on cognition: implications for understanding obesity. Proc Nutr Soc. 2017;76:455–65.
Kivipelto M, Mangialasche F, Ngandu T. Lifestyle interventions to prevent cognitive impairment, dementia and Alzheimer disease. Nat Rev Neurol. Nature Publishing Group. 2018;653–66. Available from: https://pubmed-ncbi-nlm-nih-gov.proxyau.wrlc.org/30291317/.
Neuwelt EA, Bauer B, Fahlke C, Fricker G, Iadecola C, Janigro D, et al. Engaging neuroscience to advance translational research in brain barrier biology. Nat Rev Neurosci. 2011;12:169–82. Available from: https://pubmed-ncbi-nlm-nih-gov.proxyau.wrlc.org/21331083/.
Wilhelm I, Nyul-Toth A, Suciu M, Hermenean A, Krizbai IA. Heterogeneity of the blood-brain barrier. Tissue Barriers. 2016;4:e1143544.
Kanoski SE, Zhang Y, Zheng W, Davidson TL. The effects of a high-energy diet on hippocampal function and blood-brain barrier integrity in the rat. J Alzheimer’s Dis. IOS Press. 2010;21:207–19. Available from: https://pubmed-ncbi-nlm-nih-gov.proxyau.wrlc.org/20413889/.
Davidson TL, Hargrave SL, Swithers SE, Sample CH, Fu X, Kinzig KP, et al. Inter-relationships among diet, obesity and hippocampal-dependent cognitive function. Neuroscience, 2013;253:110–22. Available from: https://pubmed-ncbi-nlm-nih-gov.proxyau.wrlc.org/23999121/.
Hargrave SL, Davidson TL, Zheng W, Kinzig KP. Western diets induce blood-brain barrier leakage and alter spatial strategies in rats. Behav Neurosci Am Psychol Assoc Inc. 2016;130:123–35. Available from: https://pubmed-ncbi-nlm-nih-gov.proxyau.wrlc.org/26595878/.
Ogata S, Ito S, Masuda T, Ohtsuki S. Changes of blood-brain barrier and brain parenchymal protein expression levels of mice under different insulin-resistance conditions induced by high-fat diet. Pharm Res. 2019;36:141.
Tsioufis C, Bafakis I, Kasiakogias A, Stefanadis C. The role of matrix metalloproteinases in diabetes mellitus. Curr Top Med Chem. 2012;12:1159–65.
Kurzepa J, Kurzepa J, Golab P, Czerska S, Bielewicz J. The significance of matrix metalloproteinase (MMP)-2 and MMP-9 in the ischemic stroke. Int J Neurosci. 2014;124:707–16.
Bronisz E, Kurkowska-Jastrzębska I. Matrix Metalloproteinase 9 in Epilepsy: The Role of Neuroinflammation in Seizure Development. Mediat Inflamm. 2016;2016:7369020.
Weekman EM, Wilcock DM. Matrix metalloproteinase in blood-brain barrier breakdown in dementia. J Alzheimers Dis. 2016;49:893–903.
Feng S, Cen J, Huang Y, Shen H, Yao L, Wang Y, et al. Matrix metalloproteinase-2 and -9 secreted by leukemic cells increase the permeability of blood-brain barrier by disrupting tight junction proteins. PLoS One. 2011;6. Available from: https://pubmed-ncbi-nlm-nih-gov.proxyau.wrlc.org/21857898/.
Rosenberg GA, Yang Y. Vasogenic edema due to tight junction disruption by matrix metalloproteinases in cerebral ischemia. Neurosurg Focus. 2007. Available from: https://pubmed-ncbi-nlm-nih-gov.proxyau.wrlc.org/17613235/.
Mazzoli A, Spagnuolo MS, Gatto C, Nazzaro M, Cancelliere R, Crescenzo R, et al. Adipose tissue and brain metabolic responses to western diet—is there a similarity between the two? Int J Mol Sci MDPI AG. 2020;21. Available from: /pmc/articles/PMC7036881/.
Sung PS, Lin PY, Liu CH, Su HC, Tsai KJ. Neuroinflammation and neurogenesis in alzheimer’s disease and potential therapeutic approaches. Int J Mol Sci MDPI AG. 2020. Available from: https://pubmed-ncbi-nlm-nih-gov.proxyau.wrlc.org/31973106/.
Gutierrez A, Vitorica J. Toward a New Concept of Alzheimer’s Disease Models: A Perspective from Neuroinflammation. J Alzheimer’s Dis. IOS Press. 2018;S329–38. Available from: https://pubmed-ncbi-nlm-nih-gov.proxyau.wrlc.org/29562520/.
Jais A, Solas M, Backes H, Chaurasia B, Kleinridders A, Theurich S, et al. Myeloid-cell-derived VEGF maintains brain glucose uptake and limits cognitive impairment in obesity. Cell. 2016;165:882–95. Available from: https://pubmed-ncbi-nlm-nih-gov.proxyau.wrlc.org/27133169/.
Nakandakari SCBR, Muñoz VR, Kuga GK, Gaspar RC, Sant’Ana MR, Pavan ICB, et al. Short-term high-fat diet modulates several inflammatory, ER stress, and apoptosis markers in the hippocampus of young mice. Brain Behav Immun. 2019;79:284–93. Available from: https://pubmed-ncbi-nlm-nih-gov.proxyau.wrlc.org/30797044/.
Alghamdi BS. The Effect of Short-Term Feeding of a High-Coconut Oil or High-Fat Diet on Neuroinflammation and the Performance of an Object–Place Task in Rats. Neurochem Res. 2021;46:287–98. Available from: https://pubmed-ncbi-nlm-nih-gov.proxyau.wrlc.org/33221998/.
Berriman J, Stevenson RJ, Thayer ZC, Thompson E, Mohamed A, Watson JDG, et al. Testing the importance of the Medial Temporal Lobes in human interoception: Does it matter if there is a memory component to the task? Neuropsychologia. 2016;91:371–9. Available from: https://pubmed-ncbi-nlm-nih-gov.proxyau.wrlc.org/27609126/.
Taylor ZB, Stevenson RJ, Ehrenfeld L, Francis HM. The Impact of Saturated Fat, Added Sugar and their Combination on Human Hippocampal Integrity and Function: A Systematic Review and Meta-analysis. Neurosci Biobehav Rev. 2021;130:91–106. Available from: https://pubmed-ncbi-nlm-nih-gov.proxyau.wrlc.org/34400179/.
Attuquayefio T, Stevenson RJ, Boakes RA, Oaten MJ, Yeomans MR, Mahmut M, et al. A high-fat high-sugar diet predicts poorer hippocampal-related memory and a reduced ability to suppress wanting under satiety. J Exp Psychol Anim Learn Cogn. 2016;42:415–28.
Attuquayefio T, Stevenson RJ, Oaten MJ, Francis HM. A four-day Western-style dietary intervention causes reductions in hippocampal-dependent learning and memory and interoceptive sensitivity. PLoS One. Public Library of Science. 2017;12.
Stevenson RJ, Francis HM, Attuquayefio T, Gupta D, Yeomans MR, Oaten MJ, et al. Hippocampal-dependent appetitive control is impaired by experimental exposure to a Western-style diet. R Soc Open Sci. 2020;7:191338.
Dossani RH, Missios S, Nanda A. The legacy of Henry Molaison (1926-2008) and the impact of his bilateral mesial temporal lobe surgery on the study of human memory. World Neurosurg. Elsevier Inc. 2015;84:1127–35.
Scoville WB, Milner B. Loss of recent memory after bilateral hippocampal lesions. J Neurol Neurosurg Psychiatry. 1957;20:11–21.
Higgs S, Williamson AC, Rotshtein P, Humphreys GW. Sensory-specific satiety is intact in amnesics who eat multiple meals: Research report. Psychol Sci. 2008;19:623–8. Available from: https://pubmed-ncbi-nlm-nih-gov.proxyau.wrlc.org/18727773/.
Rozin P, Dow S, Moscovitch M, Rajaram S. What causes humans to begin and end a meal? A role for memory for what has been eaten, as evidenced by a study of multiple meal eating in amnesic patients. Psychol Sci. 1998;9:392–6. Available from: file:///C:/Documents and Settings/office.ARTHUR/My Documents/EndNoteProject/TerryArticles/CD2/Hippocampal Grant PDFs/Hippocampus and energy regulation/Eating behavior and Hippocampus/What causes humans to begin and end a meal.pdf
Corkin S. What’s new with the amnesic patient H.M.? Nat Rev Neurosci. 2002;3:153–60. Available from: http://www.ncbi.nlm.nih.gov/pubmed/11836523.
Zuniga A, Stevenson RJ, Thayer ZC, Miller LA, Francis HM, Saluja S, et al. The impact of hippocampal damage on appetitive control. Neurocase. 2020;26:305–12. Available from: https://pubmed-ncbi-nlm-nih-gov.proxyau.wrlc.org/32894035/.
Tanaka H, Gourley DD, Dekhtyar M, Haley AP. Cognition, Brain Structure, and Brain Function in Individuals with Obesity and Related Disorders. Curr Obes Rep. 2020;9:544–9. Available from: https://pubmed-ncbi-nlm-nih-gov.proxyau.wrlc.org/33064270/.
van de Rest O, Berendsen AAM, Haveman-Nies A, de Groot LCPGM. Dietary patterns, cognitive decline, and dementia: A systematic review. Adv Nutr. 2015;6:154–68. Available from: https://pubmed-ncbi-nlm-nih-gov.proxyau.wrlc.org/25770254/.
Milte CM, Ball K, Crawford D, McNaughton SA. Diet quality and cognitive function in mid-aged and older men and women. BMC Geriatr. 2019;19. Available from: https://pubmed-ncbi-nlm-nih-gov.proxyau.wrlc.org/31864295/.
Dominguez LJ, Barbagallo M, Muñoz-Garcia M, Godos J, Martinez-Gonzalez MA. Dietary Patterns and Cognitive Decline: key features for prevention. Curr Pharm Des. 2019;25:2428–42. Available from: https://pubmed-ncbi-nlm-nih-gov.proxyau.wrlc.org/31333085/.
Francis HM, Stevenson RJ. Higher reported saturated fat and refined sugar intake is associated with reduced hippocampal-dependent memory and sensitivity to interoceptive signals. Behav Neurosci. 2011;125:943–55.
Stevenson RJ, Francis HM, Attuquayefio T, Gupta D, Yeomans MR, Oaten MJ, et al. Hippocampal-dependent appetitive control is impaired by experimental exposure to a Western-style diet. R Soc Open Sci. 2020;7. Available from: https://doi.org/10.1098/rsos.191338.
Jacka FN, Cherbuin N, Anstey KJ, Sachdev P, Butterworth P. Western diet is associated with a smaller hippocampus: a longitudinal investigation. BMC Med. 2015;13:215.
Francis HM, Stevenson RJ. Higher reported saturated fat and refined sugar intake is associated with reduced hippocampal-dependent memory and sensitivity to interoceptive signals. Behav Neurosci. 2011;125:943–55. Available from: https://pubmed-ncbi-nlm-nih-gov.proxyau.wrlc.org/22023100/.
Donato F, Alberini CM, Amso D, Dragoi G, Dranovsky A, Newcombe NS. The ontogeny of hippocampus-dependent memories. J Neurosci. 2021;41:920–6. Available from: https://pubmed-ncbi-nlm-nih-gov.proxyau.wrlc.org/33328296/.
Berridge KC. Food reward: Brain substrates of wanting and liking. Neurosci Biobehav Rev Elsevier Ltd. 1996;20:1–25.
Kanoski SE, Davidson TL. Western diet consumption and cognitive impairment: Links to hippocampal dysfunction and obesity. Physiol Behav. 2011;103:59–68. Available from: https://pubmed-ncbi-nlm-nih-gov.proxyau.wrlc.org/21167850/.
Davidson TL, Kanoski SE, Walls EK, Jarrard LE. Memory inhibition and energy regulation. Physiol Behav. 2005;86:731–46. Available from: https://www.ncbi.nlm.nih.gov/pubmed/16263144.
Abbott KN, Arnott CK, Westbrook RF, Tran DMD. The effect of high fat, high sugar, and combined high fat-high sugar diets on spatial learning and memory in rodents: A meta-analysis. Neurosci Biobehav Rev. 2019;107:399–421. Available from: https://pubmed.ncbi.nlm.nih.gov/31454627/.
Pilly PK, Howard MD, Bhattacharyya R. Modeling contextual modulation of memory associations in the hippocampus. Front Hum Neurosci. 2018;12. Available from: https://pubmed-ncbi-nlm-nih-gov.proxyau.wrlc.org/30473660/.
Garelick MG, Storm DR. The relationship between memory retrieval and memory extinction. Proc Natl Acad Sci U S A. 2005;102:9091–2. Available from: https://pubmed-ncbi-nlm-nih-gov.proxyau.wrlc.org/15967979/.
Khan NA, Baym CL, Monti JM, Raine LB, Drollette ES, Scudder MR, et al. Central Adiposity is negatively associated with hippocampal-dependent relational memory among overweight and obese children. J Pediatr. Elsevier Inc. 2015;166:302-308.e1. Available from: http://linkinghub.elsevier.com/retrieve/pii/S0022347614009536.
Khan NA, Raine LB, Drollette ES, Scudder MR, Hillman CH. The relation of saturated fats and dietary cholesterol to childhood cognitive flexibility. Appetite. 2015;93:51–6. Available from: https://pubmed-ncbi-nlm-nih-gov.proxyau.wrlc.org/25865659/.
Więckowska-Gacek A, Mietelska-Porowska A, Wydrych M, Wojda U. Western diet as a trigger of Alzheimer’s disease: From metabolic syndrome and systemic inflammation to neuroinflammation and neurodegeneration. Ageing Res Rev. 2021;70. Available from: https://pubmed-ncbi-nlm-nih-gov.proxyau.wrlc.org/34214643/.
Baranowski BJ, Marko DM, Fenech RK, Yang AJT, Macpherson REK. Healthy brain, healthy life: A review of diet and exercise interventions to promote brain health and reduce alzheimer’s disease risk1. Appl Physiol Nutr Metab. 2020;45:1055–65. Available from: https://pubmed-ncbi-nlm-nih-gov.proxyau.wrlc.org/32717151/.
Allès B, Samieri C, Jutand MA, Carmichael PH, Shatenstein B, Gaudreau P, et al. Nutrient patterns, cognitive function, and decline in older persons: Results from the three-city and nuage studies. Nutrients. 2019;11. Available from: https://pubmed-ncbi-nlm-nih-gov.proxyau.wrlc.org/31387312/.
Kendig MD, Leigh SJ, Morris MJ. Unravelling the impacts of western-style diets on brain, gut microbiota and cognition. Neurosci Biobehav Rev. 2021 [cited 2021 Aug 29];128:233–43. Available from: https://pubmed-ncbi-nlm-nih-gov.proxyau.wrlc.org/34153343/.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
The authors did not receive support from any organization for the submitted work and they declare that they have no conflict of interest.
Additional information
Publisher's note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
About this article
Cite this article
Davidson, T.L., Stevenson, R.J. Appetitive interoception, the hippocampus and western-style diet. Rev Endocr Metab Disord 23, 845–859 (2022). https://doi.org/10.1007/s11154-021-09698-2
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11154-021-09698-2