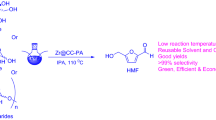
Abstract
In this research, zirconium compounds were prepared as water-tolerant solid base catalysts by a simple method. Their catalytic activities were investigated in the aqueous glucose–fructose isomerization reaction. The zirconium carbonate (ZrC) catalyst could work in wide range of reaction temperature (80–140 °C) and the maximum glucose conversion reached 45 % at 120 °C with 76 % selectivity to fructose. The ZrC catalyst was found to retain its activity without significant decrease in the fructose yield after being used for five times. In the one-pot transformation of glucose to levulinic acid (LA), the ZrC could afford 17 % yield of LA after 12 h reaction in water–toluene biphasic solvents in combination with a solid acid catalyst, Amberlyst-15. The proposed reaction system in water–toluene biphasic solvents occurred faster and gave higher LA yield than that in pure water solvent.







Similar content being viewed by others
Notes
The use of water/toluene biphasic solvent accelerated acid-catalyzed reactions since fructose yield in the initial 20 min did not change in both solvents (pure water and water/toluene). In biphasic solvent system, catalysts and sugars were distributed in water phase, while formed HMF moved into toluene phase. These phenomena pushed dehydration reaction of fructose in water phase to shift toward HMF formation [44, 45]. The utilization of pure organic solvents for transform of sugars to LA is not necessary to be investigated because the rehydration of HMF to LA is prohibited in the absence of water.
References
Corma A, Iborra S, Velty A (2007) Chem Rev 107:2411
Zakrzewska ME, Lukasik EB, Lukasik RB (2011) Chem Rev 111:397
Escobar JC, Lora ES, Venturini OJ, Yanez EE, Castillo EF, Almazan O (2009) Renew Sustain Energy Rev 13:1275
Sanders JPM, Clark JH, Harmsen GJ, Heeres HJ, Heijnen JJ, Kersten SRA, van Swaaij WPM, Moulijn JA (2012) Chem Eng Process 51:117
Tong X, Ma Y, Li Y (2010) Appl Catal A Gen 385:1
Ruiz JCS, Pineda A, Balu AM, Luque R, Campelo JM, Romero AA, Fernandez JMR (2012) Catal Today 195:162
Takagaki A, Ohara M, Nishimura S, Ebitani K (2009) Chem Commun 6276. doi:10.1039/b914087e
Ohara M, Takagaki A, Nishimura S, Ebitani K (2010) Appl Catal A Gen 383:149
Beckerle K, Okuda J (2012) J Mol Catal A Chem 356:158
Jow J, Rorrer GL, Hawley MC, Lamport DTA (1987) Biomass 14:185
Zeng W, Cheng D, Zhang H, Chen F, Zhan X (2010) React Kinet Mech Catal 100:377
Son PA, Nishimura S, Ebitani K (2012) React Kinet Mech Catal 106:185
Tewari YB (1990) Appl Biochem Biotech 23:187
Bhosale SH, Rao MB, Deshpande VV (1996) Microbiol Rev 60:280
Zhang Y, Hidajat K, Ray AK (2004) Biochem Eng J 21:111
Kooyman C, Vellenga K, deWilt HGJ (1977) Carbohydr Res 54:33
Watanabe M, Aizawa Y, Iida T, Aida TM, Levy C, Sue K, Inomata H (2005) Carbohydr Res 340:1925
Yang BY, Montgomery R (1996) Carbohydr Res 280:27
deWit G, Kieboom APG, van Bekkum H (1979) Carbohydr Res 74:157
Rendleman JA, Hodge JE (1975) Carbohydr Res 44:155
Rendleman JA, Hodge JE (1979) Carbohydr Res 75:83
Moreau C, Durand R, Roux A, Tichit D (2000) Appl Catal A Gen 193:257
Lecomte J, Finiels A, Moreau C (2002) Starch/Starke 54:75
Yu S, Kim E, Park S, Song IK, Jung JC (2012) Catal Commun 29:63
Watanabe M, Aizawa Y, Iida T, Nishimura R, Inomata H (2005) Appl Catal A Gen 295:150
Kanie Y, Akiyama K, Iwamoto M (2011) Catal Today 178:63
Román-Leshkov Y, Moliner M, Labinger JA, Davis ME (2010) Angew Chem Int Ed 49:8954
Moliner M, Román-Leshkov Y, Davis ME (2010) PNAS 107:6164
Lima S, Dias AS, Lin Z, Brandao P, Ferreira P, Pillinger M, Rocha J, Casilda VC, Valente AA (2008) Appl Catal A Gen 339:21
Nikolla E, Román-Leshkov Y, Moliner M, Davis ME (2011) ACS Catal 1:408
Pagán-Torres Y, Wang T, Gallo JMR, Shanks BH, Dumesic JA (2012) ACS Catal 2:930
Schraufnagel RA, Rase HF (1975) Ind Eng Chem Prod Res Dev 14:40
Jow J, Rorrer GL, Hawley MC (1987) Biomass 14:185
Girisuta B, Janssen LPBM, Heeres HJ (2006) Chem Eng Res Des 84:339
Hegner J, Pereira KC, deBoef B, Lucht BL (2010) Tetrahedron Lett 51:2356
Zhang J, Wu S, Zhang H, Li B (2012) Bio Res 7:3984
Chidambaram M, Venkatesan C, Rajamohanan PR, Singh AP (2003) Appl Catal A Gen 244:27
Tao Q, Reddyb BJ, He H, Frost RL, Yuana P, Zhua J (2008) Mater Chem Phys 112:869
Sinhamahapatra A, Sutradhar N, Roy B, Pal P, Bajaj HC, Panda AB (2011) Appl Catal B Environ 103:378
Sinhamahapatra A, Sutradhar N, Roy B, Tarafdar A, Bajaj HC, Panda AB (2010) Appl Catal A Gen 385:22
Nielsen RH, Wilfing G (2000) Zirconium, zirconium compounds. Wiley-VCH Verlag GmbH & Co KGaA, Weinheim, Germany
Horvat J, Klaie B, Metelko B, Sunjic V (1985) Tetrahedron Lett 26:2111
Deng L, Li J, Lai DM, Fu Y, Guo QX (2009) Angew Chem Int Ed 48:6529
Gurbuz EI, Wettstein SG, Dumesic JA (2012) ChemSusChem 5:383
Wettstein SG, Alonso DM, Chong Y, Dumesic JA (2012) Energy Environ Sci 5:8199
Vogel AI (1956) Practical organic chemistry, 3rd edn. Longman, London
Morgan SO, Yager WA (1940) Ind Eng Chem 32:15
Acknowledgments
This work is supported by the Grant-in-Aid for Scientific Research (C) (No. 22560764) by the Ministry of Education, Culture, Sports, Science and Technology (MEXT), Japan. P.A. Son is thankful to 322 Project of Ministry of Education and Training (MOET) from Vietnam Government for the scholarship.
Author information
Authors and Affiliations
Corresponding author
Electronic supplementary material
Below is the link to the electronic supplementary material.
Rights and permissions
About this article
Cite this article
Son, P.A., Nishimura, S. & Ebitani, K. Preparation of zirconium carbonate as water-tolerant solid base catalyst for glucose isomerization and one-pot synthesis of levulinic acid with solid acid catalyst. Reac Kinet Mech Cat 111, 183–197 (2014). https://doi.org/10.1007/s11144-013-0642-6
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11144-013-0642-6