Abstract
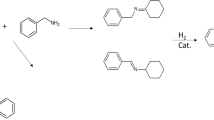
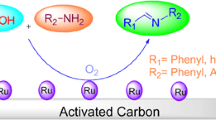
Imines were synthesized from benzyl alcohol and amines by using catalysts of gold nanoparticles supported on ZrO2 (Au/ZrO2). The effects of reaction time, temperature, gold loadings and base were investigated. High yields were achieved under moderate conditions (60 °C) in the presence of KOCH3. For instance, the yield of N-benzylidenebenzylamine produced from benzyl alcohol and benzylamine on 3 wt% Au/ZrO2 is 87 %. The synthesis of imine involves two reaction steps: selective oxidation of benzyl alcohol to benzaldehyde and the coupling reaction of amines with benzaldehyde. In the first step, the base promotes the selective oxidation. The reactions of benzyl alcohol with three different amines, aniline, n-butylamine and benzylamine, were conducted to produce corresponding imines. The results show that the amine with stronger nucleophilicity has better ability to react with benzaldehyde in the second step, resulting in higher yield of the corresponding imine. We proposed a tentative mechanism for the synthesis process.









Similar content being viewed by others
References
Hutchings GJ (2008) Chem Commun 44:1148–1164
Pina CD, Falletta E, Rossi M (2012) Chem Soc Rev 41:350–369
Aschwanden L, Mallat T, Krumeich F, Baiker A (2009) J Mol Catal A 309:57–62
Aschwanden L, Mallat T, Maciejewski M, Krumeich F, Baiker A (2010) ChemCatChem 2:666–673
So M-H, Liu Y, Ho C-M, Che C-M (2009) Chem Asian J 4:1551–1561
Zhu B, Lazar M, Trewyn B, Angelici R (2008) J Catal 260:1–6
Kegnæs S, Mielby J, Mentzel UV, Jensen T, Fristrup P, Riisager A (2012) Chem Commun 48:2427–2429
Dam JH, Osztrovszky G, Nordstrøm LU, Madsen R (2010) Chem Eur J 16:6820–6827
Ishida T, Kawakita N, Akita T, Haruta M (2009) Gold Bull 42:267–274
Grirrane A, Corma A, Garcia H (2009) J Catal 264:138–144
Lang X, Ji H, Chen C, Ma W, Zhao J (2011) Angew Chem Int Ed 50:3934–3937
Chakraborti AK, Bhagat S, Rudrawar S (2004) Tetrahedron Lett 45:7641–7644
Corma A, Leyva-Pérez A, Sabater MJ (2011) Chem Rev 111:1657–1712
Zhu B, Angelici RJ (2007) Chem Commun 42:2157–2159
Khatri PK, Jain SL, Sivakumar KLN, Sain B (2011) Org Biomol Chem 9:3370–3374
Buonomenna MG, Drioli E, Nugent WA, Prins LJ, Scrimin P, Licini G (2004) Tetrahedron Lett 45:7515–7518
Colonna S, Pironti V, Carrea G, Pasta P, Zambianchi F (2004) Tetrahedron 60:569–575
Liu L, Zhang S, Fu X, Yan C-H (2011) Chem Commun 47:10148–10150
Dhakshinamoorthy A, Alvaro M, Garcia H (2010) ChemCatChem 2:1438–1443
Zhang Y, Cui X, Shi F, Deng Y (2012) Chem Rev 112:2467–2505
Layer RW (1963) Chem Rev 63:489–510
Bahn S, Imm S, Neubert L, Zhang M, Neumann H, Beller M (2011) ChemCatChem 3:1853–1864
He J, Yamaguchi K, Mizuno N (2010) Chem Lett 39:1182–1183
Tang C-H, He L, Liu Y-M, Cao Y, He H-Y, Fan K-N (2011) Chem Eur J 17:7172–7177
Xu C, Goh LY, Pullarkat SA (2011) Organometallics 30:6499–6502
Zanardi A, Mata JA, Peris E (2010) Chem Eur J 16:10502–10506
Rigoli JW, Moyer SA, Pearce SD, Schomaker JM (2012) Org Biomol Chem 10:1746–1749
Sithambaram S, Kumar R, Son Y, Suib S (2008) J Catal 253:269–277
Kwon MS, Kim S, Park S, Bosco W, Chidrala RK, Park J (2009) J Org Chem 74:2877–2879
Kang Q, Zhang Y (2012) Green Chem 14:1016–1019
Sun H, Su F-Z, Ni J, Cao H-Y, Fan K-N (2009) Angew Chem Int Ed 48:4390–4393
Kegnæs S, Mielby J, Mentzel UV, Christensen CH, Riisager A (2010) Green Chem 12:1437–1441
Zhu H, Ke X, Yang X, Sarina S, Liu H (2010) Angew Chem Int Ed 49:9657–9661
Ishida T, Takamura R, Takei T, Akita T, Haruta M (2012) Appl Catal A 413–414:261–266
Gomez S, Peters JA, Maschmeyer T (2002) Adv Synth Catal 344:1037–1057
Liu X, Friend CM (2010) Langmuir 26:16552–16557
Xu B, Friend CM (2011) Faraday Discuss 152:307–320
Yang J, Guan Y, Verhoeven T, van Santen R, Li C, Hensen EJM (2009) Green Chem 11:322–325
Acknowledgments
Financial supports from the National Natural Science Foundation of China, No. 20966008 and Opening Project of Natural Science Foundation of Inner Mongolia, No. 2010KF02 are gratefully acknowledged.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Cui, W., Zhu, H., Jia, M. et al. One-pot synthesis of imines from benzyl alcohol and amines on Au/ZrO2 catalyst. Reac Kinet Mech Cat 109, 551–562 (2013). https://doi.org/10.1007/s11144-013-0576-z
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11144-013-0576-z