Abstract
Purpose
The primary goal of this analysis is to describe the health-related quality of life (HRQoL), medical history, and medication use among adolescents and adults individuals with Angelman syndrome (AS).
Methods
The analysis uses baseline data collected during the STARS study, a double-blind placebo controlled trial of gaboxadol (OV101) in adolescents and adults with AS. The HRQoL was estimated using EuroQoL 5-Dimension 5-Level (EQ-5D) health questionnaire proxy 1 version, which was completed by the caregivers. EQ-5D consists of two parts, a 5-dimension descriptive and a visual analogue scale (VAS) component. The utility score derived from EQ-5D ranges from 0 to 1 (perfect health) and VAS ranges from 0 to 100 (perfect health).
Results
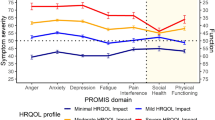
87 individuals with AS were included in the present analysis. The mean utility score was 0.44 ± 0.20 and VAS score was 84 ± 1.5. The EQ-5D data indicated that the self-care, mobility and daily activities were most impacted. All adolescents (100%) and most adults (93%) had at least moderate problems with self-care activities, such as washing or dressing themselves. More than half (55%) of the adolescents and adults had at least moderate issues with mobility and usual activities. Approximately, 30% of adolescents and adults had moderate to extreme problems with anxiety/depression. High baseline concomitant use of medications was observed across both age groups with an average of 5 medications being used per person.
Conclusion
This study highlights the impact of AS on HRQoL and medication utilization among adolescents and adults individuals with AS.


Similar content being viewed by others
Data availability
Ovid Therapeutics Inc (Ovid) is committed to providing qualified scientific researchers appropriate access to anonymized data and clinical study information from the company’s clinical trials for the purpose of conducting legitimate scientific research. Additionally, Ovid shall post all company-sponsored safety and efficacy studies of clinical trial participants on the clinical trial registries of the U.S. National Institutes of Health (NIH) and European Medicines Agency (EMA), as mandated by applicable laws and regulations. Ovid supports an approach to sharing data that responsibly reflects the interests of all parties involved in clinical trials, including protecting (i) the rights and privacy of trial participants, (ii) the innovator’s intellectual property rights, and (iii) other incentives for innovation, and as such, will evaluate requests for sharing company clinical trial data with qualified external scientific researchers. Data will be made available for request either (i) after product approval in the United States and European Union, (ii) after product development is discontinued or (iii) as otherwise required by law or regulation. There are circumstances that may prevent Ovid from sharing the requested data as the product is still investigational at this time.
Code availability
Not applicable.
References
Williams, C. A., Beaudet, A. L., Clayton-Smith, J., Knoll, J. H., Kyllerman, M., Laan, L. A., Magenis, R. E., Moncla, A., Schinzel, A. A., Summers, J. A., & Wagstaff, J. (2006). Angelman syndrome 2005: updated consensus for diagnostic criteria. American Journal of Medical Genetics Part A, 140(5), 413–8.
Bindels-de Heus, K. G., Mous, S. E., ten Hooven-Radstaake, M., van Iperen-Kolk, B. M., Navis, C., Rietman, A. B., Ten Hoopen, L. W., Brooks, A. S., ENCORE Expertise Center for AS, Elgersma, Y., & Moll, H. A. (2020). An overview of health issues and development in a large clinical cohort of children with Angelman syndrome. American Journal of Medical Genetics Part A, 182(1), 53–63.
Buiting, K., Williams, C., & Horsthemke, B. (2016). Angelman syndrome - insights into a rare neurogenetic disorder. Nature Reviews. Neurology, 12(10), 584–593.
Bird, L. M. (2014). Angelman syndrome: Review of clinical and molecular aspects. The Application of Clinical Genetics, 7, 93–104.
Guerrini, R., Carrozzo, R., Rinaldi, R., & Bonanni, P. (2003). Angelman syndrome: etiology, clinical features, diagnosis, and management of symptoms. Paediatric Drugs, 5(10), 647–661.
Tan, W. H., & Bird, L. M. (2016). Angelman syndrome: Current and emerging therapies in 2016. American Journal of Medical Genetics. Part C, Seminars in Medical Genetics, 172(4), 384–401.
Wheeler, A. C., Sacco, P., & Cabo, R. (2017). Unmet clinical needs and burden in Angelman syndrome: A review of the literature. Orphanet Journal of Rare Diseases, 12, 164.
Khan, N., Cabo, R., Tan, W. H., Tayag, R., & Bird, L. M. (2019). Healthcare burden among individuals with Angelman syndrome: Findings from the Angelman syndrome natural history study. Molecular Genetics & Genomic Medicine, 7(7), e00734.
Griffith, G. M., Hastings, R. P., Oliver, C., Howlin, P., Moss, J., Petty, J., & Tunnicliffe, P. (2011). Psychological well-being in parents of children with Angelman, Cornelia de Lange and Cri du Chat syndromes. Journal of Intellectual Disability Research, 55(4), 397–410.
Willgoss, T., Cassater, D., Connor, S., Krishnan, M. L., Miller, M. T., Dias-Barbosa, C., Phillips, D., McCormack, J., Bird, L. M., Burdine, R. D., & Claridge, S. (2021). Measuring what matters to individuals with Angelman syndrome and their families: development of a patient-centered disease concept model. Child Psychiatry & Human Development. https://doi.org/10.1007/s10578-020-01051-z
Larson, A. M., Shinnick, J. E., Shaaya, E. A., Thiele, E. A., & Thibert, R. L. (2015). Angelman syndrome in adulthood. American Journal of Medical Genetics Part A, 167A(2), 331–344.
Adams, D., Clarke, S., Griffith, G., Howlin, P., Moss, J., Petty, J., Tunnicliffe, P., & Oliver, C. (2018). Mental health and well-being in mothers of children with rare genetic syndromes showing chronic challenging behavior: A cross-sectional and longitudinal study. American Journal on Intellectual and Developmental Disabilities, 123(3), 241–253.
Grieco, J. C., Romero, B., Flood, E., Cabo, R., & Visootsak, J. (2019). Conceptual model of Angelman syndrome and review of relevant clinical outcomes assessments (COAs). Patient, 12(1), 97–112.
Khan, N., Cabo, R., Tan, W. H., Tayag, R., & Bird, L. M. (2019). An observational study of pediatric healthcare burden in Angelman syndrome: results from a real-world study. Orphanet Journal of Rare Diseases, 14, 239.
Thomson, A. K., Glasson, E. J., & Bittles, A. H. (2006). A long-term population-based clinical and morbidity profile of Angelman syndrome in Western Australia: 1953–2003. Disability and Rehabilitation, 28(5), 299–305.
Domínguez-Berjón, M. F., Zoni, A. C., Esteban-Vasallo, M. D., Sendra-Gutiérrez, J. M., & Astray-Mochales, J. (2018). Main causes of hospitalization in people with Angelman syndrome. Journal of Applied Research in Intellectual Disabilities, 31(3), 466–469.
Bird, L. M., Ochoa-Lubinoff, C., Tan, W. H., Heimer, G., Melmed, R. D., Rakhit, A., Visootsak, J., During, M. J., Holcroft, C., Burdine, R. D., & Kolevzon, A. (2021). The stars phase 2 study: A randomized controlled trial of gaboxadol in Angelman syndrome. Neurology. https://doi.org/10.1212/WNL.0000000000011409
Herdman, M., Gudex, C., Lloyd, A., Janssen, M. F., Kind, P., Parkin, D., Bonsel, G., & Badia, X. (2011). Development and preliminary testing of the new five-level version of EQ-5D (EQ-5D-5L). Quality of Life Research, 20(10), 1727–36.
Dolan, P. (1997). Modeling valuations for EuroQol health states. Medical Care, 35(11), 1095–1108.
Pickard, A. S., Law, E. H., Jiang, R., Pullenayegum, E., Shaw, J. W., Xie, F., Oppe, M., Boye, K. S., Chapman, R. H., Gong, C. L., & Balch, A. (2019). United States valuation of EQ-5D-5L health states using an international protocol. Value in Health, 22(8), 931–941.
Shaw, J. W., Johnson, J. A., & Coons, S. J. (2005). US valuation of the EQ-5D health states: Development and testing of the D1 valuation model. Medical Care, 43(3), 203–220.
Euroquol, G. (1990). EuroQol–a new facility for the measurement of health-related quality of life. Health Policy, 16(3), 199–208.
Janssen, B., & Szende, A. (2014). Population Norms for the EQ-5D. Self-Reported Population Health: An International Perspective based on EQ-5D, in Self-Reported Population Health: An International Perspective based on EQ-5D. Springer.
Jiang, R., Janssen, M. F. B., & Pickard, A. S. (2021). US population norms for the EQ-5D-5L and comparison of norms from face-to-face and online samples. Quality of Life Research, 30(3), 803–816.
Chevreul, K., Gandré, C., Brigham, K. B., López-Bastida, J., Linertová, R., Oliva-Moreno, J., Serrano-Aguilar, P., Posada-de-la-Paz, M., Taruscio, D., Schieppati, A., & Iskrov, G. (2016). Social/economic costs and health-related quality of life in patients with fragile X syndrome in Europe. The European Journal of Health Economics, 17(Suppl 1), 43–52.
Cavazza, M., et al. (2016). Social/economic costs and health-related quality of life in patients with Duchenne muscular dystrophy in Europe. The European Journal of Health Economics, 17(Suppl 1), 19–29.
Lin, J., Wong, C. K. H., Cheung, J. P. Y., Cheung, P. W. H., & Luo, N. (2022). Psychometric performance of proxy-reported EQ-5D youth version 5-level (EQ-5D-Y-5L) in comparison with three-level (EQ-5D-Y-3L) in children and adolescents with scoliosis. The European Journal of Health Economics, 23(8), 1383–95.
Takura, T., Koike, T., Matsuo, Y., Sekimoto, A., & Mutou, M. (2022). Proxy responses regarding quality of life of patients with terminal lung cancer: preliminary results from a prospective observational study. BMJ Open, 12(2), e048232.
Kelly, C., Hulme, C., Graham, L., Ellwood, A., Patel, I., Cundill, B., Farrin, A., Goodwin, M., Hull, K., Fisher, J., & Forster, A. (2021). Inter-rater reliability of care home staff’s proxy judgements with residents’ assessments of their own health-related quality of life: an analysis of the PATCH trial EQ-5D data. Age Ageing, 50(4), 1314–1320.
Fitriana, T. S., Purba, F. D., Stolk, E., & Busschbach, J. J. (2022). EQ-5D-Y-3L and EQ-5D-Y-5L proxy report: psychometric performance and agreement with self-report. Health and Quality of Life Outcomes, 20(1), 88.
Balboni, G., Coscarelli, A., Giunti, G., & Schalock, R. L. (2013). The assessment of the quality of life of adults with intellectual disability: The use of self-report and report of others assessment strategies. Research in Developmental Disabilities., 34(11), 4248–4254.
Lamsal, R., Finlay, B., Whitehurst, D. G., & Zwicker, J. D. (2020). Generic preference-based health-related quality of life in children with neurodevelopmental disorders: a scoping review. Developmental Medicine & Child Neurology, 62(2), 169–177.
Péntek, M., Gulácsi, L., Brodszky, V., Baji, P., Boncz, I., Pogány, G., López-Bastida, J., Linertová, R., Oliva-Moreno, J., Serrano-Aguilar, P., & Posada-de-la-Paz, M. (2016). Social/economic costs and health-related quality of life of mucopolysaccharidosis patients and their caregivers in Europe. The European Journal of Health Economics, 17(Suppl 1), 89–98.
López-Bastida, J., Linertová, R., Oliva-Moreno, J., Posada-de-la-Paz, M., Serrano-Aguilar, P., Kanavos, P., Taruscio, D., Schieppati, A., Iskrov, G., Baji, P., & Delgado, C. (2016). Social/economic costs and health-related quality of life in patients with Prader-Willi syndrome in Europe. The European Journal of Health Economics, 17(Suppl 1), 99–108.
Acknowledgements
The authors thank the patients in the STARS study, their families and the STARS investigators and participating study centres. Statistical assistance in the preparation of this manuscript was provided by Maria Reynolds and Beth Sherrill at RTI Health Solutions. Beth Sherrill also reviewed and provided comments on the first draft of the manuscript. Financial support was from Ovid. Editorial support was provided by Shalaka Samant, Ph.D. from RWEC, LLC.
Funding
This study was funded by Ovid Therapeutics Inc, New York, NY, USA.
Author information
Authors and Affiliations
Consortia
Contributions
All authors read and approved the final manuscript. All authors helped interpret the data and contributed to the manuscript. NK designed the analyses, interpreted the data and wrote the manuscript.
Corresponding author
Ethics declarations
Competing interests
Dr. Khan is a paid consultant for Ovid Therapeutics. Ms. Cabo is an employee of Ovid Therapeutics. Dr. Burdine consults on clinical trials supported by Ovid Therapeutics. Dr. Tan has participated in clinical trials supported by Ovid Therapeutics and Dimension Therapeutics and has also received research support from Ovid Therapeutics. Dr. Keary has consulted for Ovid Therapeutics and is participating in clinical trials supported by Ovid Therapeutics. Dr. Ochoa-Lubinoff has consulted for Ovid Therapeutics and participated in clinical trials supported by Ovid Therapeutics. Dr. Bird is participating in clinical trials supported by Ovid Therapeutics.
Ethical approval
Protocol approval and ethics oversight was provided by the regional IRBs/ECs at each site. Written informed consent was obtained from the legally authorized representative of each participating individual, prior to any study procedures.
Consent for publication
Not applicable.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Springer Nature or its licensor (e.g. a society or other partner) holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Khan, N., Cabo, R., Burdine, R.D. et al. Health-related quality of life and medication use among individuals with Angelman syndrome. Qual Life Res 32, 2059–2067 (2023). https://doi.org/10.1007/s11136-023-03375-4
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11136-023-03375-4