Abstract
Background
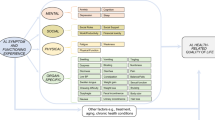
We conducted a cross-sectional study to characterize health-related quality of life and symptom burden in individuals living with light chain (AL) amyloidosis.
Methods
Members of the Amyloidosis Support Groups, Inc. with AL amyloidosis who consented to this IRB-approved survey provided information on their amyloidosis diagnosis, treatment, symptoms, and functioning. HRQL was measured using PROMIS and PRO-CTCAE questionnaires.
Results
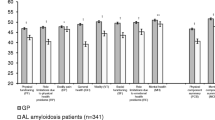
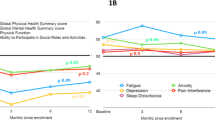
Among 297 participants who responded, the median age at diagnosis was 60 years (23–82) with 52% female and 90% white race. There were 69% AL (lambda) and 39% reported 3 or more organs involved with amyloidosis (58% cardiac, 58% renal, 30% neurological AL). Time from diagnosis was less than 2 years in 64 (22%), 2–5 years in 105 (36%), > 5 years in 126 (43%), and unknown in 2 (< 1%) individuals. Therapy included prior chemotherapy in 88% and stem cell transplant in 52%. Fifty percent of the cohort was on active treatment. Multiple domains were impaired in AL amyloidosis compared to the general population, including physical function, fatigue, and social roles. While highest among those within 2 years of diagnosis, high symptom burden was also seen in long-term survivors. A trend to decreased severity and number of impaired symptoms was seen with longer treatment-free interval but many symptoms remained persistent.
Conclusions
Significant and persistent symptom burden is seen in AL amyloidosis. Patient-reported outcomes should be routinely measured and used to provide best supportive care to all AL amyloidosis patients, including long-term survivors and those not on active therapy.


Similar content being viewed by others
Data availability
Data will be shared upon reasonable request to the corresponding author.
References
Bayliss, M., McCausland, K. L., Guthrie, S. D., & White, M. K. (2017). The burden of amyloid light chain amyloidosis on health-related quality of life. Orphanet Journal of Rare Diseases, 12(1), 15. https://doi.org/10.1186/s13023-016-0564-2
Quock, T. P., Yan, T., Chang, E., Guthrie, S., & Broder, M. S. (2018). Epidemiology of AL amyloidosis: A real-world study using US claims data. Blood Advances, 2(10), 1046–1053. https://doi.org/10.1182/bloodadvances.2018016402
Palladini, G., Milani, P., & Merlini, G. (2020). Management of AL amyloidosis in 2020. Blood, 136(23), 2620–2627. https://doi.org/10.1182/blood.2020006913
Merlini, G. (2017). AL amyloidosis: From molecular mechanisms to targeted therapies. Hematology/the Education Program of the American Society of Hematology American Society of Hematology Education Program. https://doi.org/10.1182/asheducation-2017.1.1
D’Souza, A., Myers, J., Cusatis, R., et al. (2022). Development of a conceptual model of patient-reported outcomes in light chain amyloidosis: A qualitative study. Quality of Life Research, 31(4), 1083–1092. https://doi.org/10.1007/s11136-021-02943-w
Rizio, A. A., White, M. K., McCausland, K. L., et al. (2018). Treatment tolerability in patients with immunoglobulin light-chain amyloidosis. Am Health Drug Benefits, 11(8), 430–437.
Lin, H. M., Seldin, D., Hui, A. M., Berg, D., Dietrich, C. N., & Flood, E. (2015). The patient’s perspective on the symptom and everyday life impact of AL amyloidosis. Amyloid : The International Journal of Experimental and Clinical Investigation : The Official Journal of the International Society of Amyloidosis, 22(4), 244–251. https://doi.org/10.3109/13506129.2015.1102131
Sanchorawala, V., McCausland, K. L., White, M. K., et al. (2017). A longitudinal evaluation of health-related quality of life in patients with AL amyloidosis: Associations with health outcomes over time. British Journal of Haematology, 179(3), 461–470. https://doi.org/10.1111/bjh.14889
Warsame, R., & D’Souza, A. (2019). Patient reported outcomes have arrived: A practical overview for clinicians in using patient reported outcomes in oncology. Mayo Clinic Proceedings, 94(11), 2291–2301. https://doi.org/10.1016/j.mayocp.2019.04.005
Warsame, R., Kumar, S. K., Gertz, M. A., et al. (2017). Hematology patient reported symptom screen to assess quality of life for AL amyloidosis. American Journal of Hematology. https://doi.org/10.1002/ajh.24676
D’Souza, A., Magnus, B. E., Myers, J., Dispenzieri, A., & Flynn, K. E. (2020). The use of PROMIS patient-reported outcomes (PROs) to inform light chain (AL) amyloid disease severity at diagnosis. Amyloid : the International Journal of Experimental and Clinical Investigation : the Official Journal of the International Society of Amyloidosis. https://doi.org/10.1080/13506129.2020.1713743
D’Souza, A., Brazauskas, R., Dispenzieri, A., Panepinto, J., & Flynn, K. E. (2021). Changes in patient-reported outcomes in light chain amyloidosis in the first year after diagnosis and relationship to NT-proBNP change. Blood Cancer Journal, 11(2), 29. https://doi.org/10.1038/s41408-021-00412-8
McCausland, K. L., Quock, T. P., Rizio, A. A., et al. (2018). Cardiac biomarkers and health-related quality of life in patients with light chain (AL) amyloidosis. British Journal of Haematology. https://doi.org/10.1111/bjh.15693
Basch, E., Deal, A. M., Dueck, A. C., et al. (2017). Overall survival results of a trial assessing patient-reported outcomes for symptom monitoring during routine cancer treatment. JAMA : The journal of the American Medical Association, 318(2), 197–198. https://doi.org/10.1001/jama.2017.7156
Rothrock, N. E., Hays, R. D., Spritzer, K., Yount, S. E., Riley, W., & Cella, D. (2010). Relative to the general US population, chronic diseases are associated with poorer health-related quality of life as measured by the patient-reported outcomes measurement information system (PROMIS). Journal of Clinical Epidemiology, 63(11), 1195–1204. https://doi.org/10.1016/j.jclinepi.2010.04.012
Hays, R. D., Bjorner, J. B., Revicki, D. A., Spritzer, K. L., & Cella, D. (2009). Development of physical and mental health summary scores from the patient-reported outcomes measurement information system (PROMIS) global items. Quality of Life Research : An International Journal of Quality of Life Aspects of Treatment, Care and Rehabilitation, 18(7), 873–880. https://doi.org/10.1007/s11136-009-9496-9
White, M. K., McCausland, K. L., Sanchorawala, V., Guthrie, S. D., & Bayliss, M. S. (2017). Psychometric validation of the SF-36 Health Survey in light chain amyloidosis: Results from community-based and clinic-based samples. Patient Relat Outcome Meas, 8, 157–167. https://doi.org/10.2147/PROM.S146849
Brodke, D. S., Goz, V., Voss, M. W., Lawrence, B. D., Spiker, W. R., & Man, H. (2016). PROMIS(R) PF CAT Outperforms the ODI and SF-36 Physical function domain in spine patients. Spine. https://doi.org/10.1097/BRS.0000000000001965
Oude Voshaar, M. A., Ten Klooster, P. M., Glas, C. A., et al. (2015). Validity and measurement precision of the PROMIS physical function item bank and a content validity-driven 20-item short form in rheumatoid arthritis compared with traditional measures. Rheumatology, 54(12), 2221–2229. https://doi.org/10.1093/rheumatology/kev265
Hays, R. D., Spritzer, K. L., Fries, J. F., & Krishnan, E. (2015). Responsiveness and minimally important difference for the patient-reported outcomes measurement information system (PROMIS) 20-item physical functioning short form in a prospective observational study of rheumatoid arthritis. Annals of the Rheumatic Diseases, 74(1), 104–107. https://doi.org/10.1136/annrheumdis-2013-204053
Reeve, B. B., Mitchell, S. A., Dueck, A. C., et al. (2014). Recommended patient-reported core set of symptoms to measure in adult cancer treatment trials. Journal of the National Cancer Institute. https://doi.org/10.1093/jnci/dju129
Ihne, S., Morbach, C., Obici, L., Palladini, G., & Stork, S. (2019). Amyloidosis in heart failure. Current Heart Failure Reports, 16(6), 285–303. https://doi.org/10.1007/s11897-019-00446-x
Cella, D., Riley, W., Stone, A., et al. (2010). The patient-reported outcomes measurement information system (PROMIS) developed and tested its first wave of adult self-reported health outcome item banks: 2005–2008. Journal of Clinical Epidemiology, 63(11), 1179–1194. https://doi.org/10.1016/j.jclinepi.2010.04.011
Dewitt, B., Feeny, D., Fischhoff, B., et al. (2018). Estimation of a preference-based summary score for the patient-reported outcomes measurement information system: the PROMIS((R))-preference (PROPr) scoring system. Medical Decision Making : An International Journal of the Society for Medical Decision Making, 38(6), 683–698. https://doi.org/10.1177/0272989X18776637
Basch, E., Reeve, B. B., Mitchell, S. A., et al. (2014). Development of the national cancer institute’s patient-reported outcomes version of the common terminology criteria for adverse events (PRO-CTCAE). Journal of the National Cancer Institute. https://doi.org/10.1093/jnci/dju244
HealthMeasures Scoring Instructions. http://www.healthmeasures.net/score-and-interpret/calculate-scores/scoring-instructions
Chen, C. X., Kroenke, K., Stump, T., et al. (2019). Comparative responsiveness of the PROMIS pain interference short forms with legacy pain measures: Results from three randomized clinical trials. The Journal of Pain, 20(6), 664–675. https://doi.org/10.1016/j.jpain.2018.11.010
Yost, K. J., Eton, D. T., Garcia, S. F., & Cella, D. (2011). Minimally important differences were estimated for six patient-reported outcomes measurement information system-cancer scales in advanced-stage cancer patients. Journal of Clinical Epidemiology, 64(5), 507–516. https://doi.org/10.1016/j.jclinepi.2010.11.018
Terwee, C. B., Peipert, J. D., Chapman, R., et al. (2021). Minimal important change (MIC): A conceptual clarification and systematic review of MIC estimates of PROMIS measures. Quality of Life Research : An International Journal of Quality of Life Aspects of Treatment, Care and Rehabilitation, 30(10), 2729–2754. https://doi.org/10.1007/s11136-021-02925-y
Basch, E., Becker, C., Rogak, L. J., et al. (2021). Composite grading algorithm for the national cancer institute’s patient-reported outcomes version of the common terminology criteria for adverse events (PRO-CTCAE). Clinical Trials, 18(1), 104–114. https://doi.org/10.1177/1740774520975120
Jensen, R. E., Potosky, A. L., Moinpour, C. M., et al. (2017). United States population-based estimates of patient-reported outcomes measurement information system symptom and functional status reference values for individuals with cancer. Journal of Clinical Oncology : Official Journal of the American Society of Clinical Oncology, 35(17), 1913–1920. https://doi.org/10.1200/JCO.2016.71.4410
Staron, A., Connors, L. H., Zheng, L., Doros, G., & Sanchorawala, V. (2020). Race/ethnicity in systemic AL amyloidosis: perspectives on disease and outcome disparities. Blood Cancer Journal, 10(11), 118. https://doi.org/10.1038/s41408-020-00385-0
D’Souza, A., Pezzin, L., Laud, P., & Singh, A. (2021). Racial disparities in patients diagnosed with light chain (AL) amyloidosis. Blood Cancer Journal, 11(4), 72. https://doi.org/10.1038/s41408-021-00466-8
Wechalekar, A. D., Gillmore, J. D., & Hawkins, P. N. (2016). Systemic amyloidosis. Lancet, 387(10038), 2641–2654. https://doi.org/10.1016/S0140-6736(15)01274-X
Acknowledgements
The authors gratefully acknowledge the contribution of all participants and the Amyloidosis Support Groups, Inc. in this study.
Funding
National Heart, Lung, and Blood Institute, K23HL141445, Anita D'Souza
Author information
Authors and Affiliations
Contributions
AD: obtained funding, designed the survey, reviewed the analysis and wrote the paper, AS: conducted the data cleanup and biostatistical analysis, IA: created and maintained the dataset and assisted in data cleanup, MF: contributed to the study design and assisted with survey dissemination, KEF: designed the survey, reviewed the analysis, and edited the manuscript. All authors reviewed and approved the final draft.
Corresponding author
Ethics declarations
Conflicts of interest
AD reports institutional research funding from Abbvie, Caelum, Janssen, Prothena, Sanofi, Takeda and TeneoBio, Ad Board fees from BMS, Consulting fees from Prothena and Janssen. IA, AS, MF report no conflicts. KEF reports institutional research funding from Novartis, Consulting fees from Inhibikase and Pfizer. The authors confirm no competing financial interests in relation to the work in this manuscript.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
This work was presented in part as a poster at the XVIIIth International Symposium on Amyloidosis, Heidelberg, Germany in Sept 2022.
Rights and permissions
Springer Nature or its licensor (e.g. a society or other partner) holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
D’Souza, A., Szabo, A., Akinola, I. et al. A cross-sectional study of patient-reported outcomes and symptom burden using PROMIS and PRO-CTCAE measures in light chain amyloidosis. Qual Life Res 32, 1807–1817 (2023). https://doi.org/10.1007/s11136-023-03354-9
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11136-023-03354-9