Abstract
Purpose
Huntington disease (HD) is a chronic, debilitating genetic disease that affects physical, emotional, cognitive, and social health. Existing patient-reported outcomes (PROs) of health-related quality of life (HRQOL) used in HD are neither comprehensive, nor do they adequately account for clinically meaningful changes in function. While new PROs examining HRQOL (i.e., Neuro-QoL—Quality of Life in Neurological Disorders and PROMIS—Patient-Reported Outcomes Measurement Information System) offer solutions to many of these shortcomings, they do not include HD-specific content, nor have they been validated in HD. HDQLIFE addresses this by validating 12 PROMIS/Neuro-QoL domains in individuals with HD and by using established PROMIS methodology to develop new, HD-specific content.
Methods
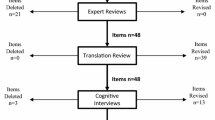
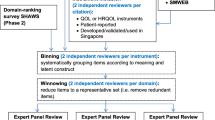
New item pools were developed using cognitive debriefing with individuals with HD, and expert, literacy, and translatability reviews. Existing item banks and new item pools were field tested in 536 individuals with prodromal, early-, or late-stage HD.
Results
Moderate to strong relationships between Neuro-QoL/PROMIS measures and generic self-report measures of HRQOL, and moderate relationships between Neuro-QoL/PROMIS and clinician-rated measures of similar constructs supported the validity of Neuro-QoL/PROMIS in individuals with HD. Exploratory and confirmatory factor analysis, item response theory, and differential item functioning analyses were utilized to develop new item banks for Chorea, Speech Difficulties, Swallowing Difficulties, and Concern with Death and Dying, with corresponding six-item short forms. A four-item short form was developed for Meaning and Purpose.
Conclusions
HDQLIFE encompasses both validated Neuro-QoL/PROMIS measures, as well as five new scales in order to provide a comprehensive assessment of HRQOL in HD.

Similar content being viewed by others
References
The Huntington’s Disease Collaborative Research Group. (1993). A novel gene containing a trinucleotide repeat that is expanded and unstable on Huntington’s disease chromosomes. Cell, 72, 971–983.
Ross, C. A., et al. (2014). Huntington disease: Natural history, biomarkers and prospects for therapeutics. Nat Rev Neurol, 10(4), 204–216.
Squitieri, F., et al. (2015). Epidemiology of Huntington disease: First post-HTT gene analysis of prevalence in Italy. Clinical Genetics, 89, 367–370.
Evans, S. J. W., et al. (2013). Prevalence of adult Huntington’s disease in the UK based on diagnoses recorded in general practice records. Journal of Neurology, Neurosurgery and Psychiatry, 84(10), 1156–1160.
Paulsen, J. S. (2010). Early detection of huntington disease. Future Neurology, 5(1), 85–104.
Ross, C. A., et al. (1997). Huntington disease and the related disorder, dentatorubral–pallidoluysian atrophy (DRPLA). Medicine (Baltimore), 76(5), 305–338.
Cella, D. F. (1995). Measuring quality of life in palliative care. Seminars in Oncology, 22(2 Suppl 3), 73–81.
World Health Organization, W. (1946). Preamble to the constitution of the World Health Organization as adopted by the International Health Conference. In International health conference, New York.
Campbell, A. J., Converse, P. E., & Rodgers, W. L. (1976). The quality of American life: Perceptions, evaluations, and satisfactions. New York: Russell Sage Foundation.
Patrick, D. L., & Erikson, P. (1988). What constitutes quality of life? Concepts and dimensions. Clinical Nutrition, 7(2), 53–63.
Jankovic, J., & Roos, R. A. (2014). Chorea associated with Huntington’s disease: To treat or not to treat? Movement Disorders, 29(11), 1414–1418.
Peavy, G. M., et al. (2010). Cognitive and functional decline in Huntington’s disease: Dementia criteria revisited. Movement Disorders, 25(9), 1163–1169.
Solomon, A. C., et al. (2007). Verbal episodic memory declines prior to diagnosis in Huntington’s disease. Neuropsychologia, 45(8), 1767–1776.
Ready, R. E., et al. (2008). Patient and caregiver quality of life in Huntington’s disease. Movement Disorders, 23(5), 721–726.
Tibben, A., et al. (1993). Presymptomatic DNA-testing for Huntington disease—Pretest attitudes and expectations of applicants and their partners in the Dutch program. American Journal of Medical Genetics, 48(1), 10–16.
Hocaoglu, M. B., Gaffan, E. A., & Ho, A. K. (2012). The Huntington’s disease health-related Quality of Life questionnaire (HDQoL): A disease-specific measure of health-related quality of life. Clinical Genetics, 81(2), 117–122.
Paulsen, J. S., et al. (2013). A review of quality of life after predictive testing for and earlier identification of neurodegenerative diseases. Progress in Neurobiology, 110, 2–28.
Cella, D., et al. (2011). The neurology quality of life measurement (Neuro-QOL) initiative. Archives of Physical Medicine and Rehabilitation, 92(Suppl 1), S28–S36.
Gershon, R. C., et al. (2012). Neuro-QOL: Quality of life item banks for adults with neurological disorders: Item development and calibrations based upon clinical and general population testing. Quality of Life Research, 21(3), 475–486.
Cella, D., et al. (2010). The Patient-Reported Outcomes Measurement Information System (PROMIS) developed and tested in its first wave of adult self-reported health outcome item banks: 2005–2008. Journal of Clinical Epidemiology, 63, 1179–1194.
van der Linden, W. J., & Hambleton, R. K. (1997). Handbook of modern item response theory. New York: Springer.
Choppin, B. (1968). Item bank using sample-free calibration. Nature, 219(5156), 870–872.
Choppin, B. (1981). Educational measurement and the item bank model. In C. Lacey & D. Lawton (Eds.), Issues in evaluation and accountability (pp. 204–221). London: Methuen.
Nance, M. A. (2007). Comprehensive care in Huntington’s disease: A physician’s perspective. Brain Research Bulletin, 72(2–3), 175–178.
Lai, J. S., et al. (2011). How item banks and its applications can influence measurement practice in rehabilitation medicine: A PROMIS fatigue item bank example. Archives of Physical Medicine and Rehabilitation, 92(Supp 1), S20–S27.
Carlozzi, N. E., et al. (2016). New measures to capture end of life concerns in Huntington disease: Meaning and purpose and concern with Death and Dying from HDQLIFE (a patient-reported outcomes measurement system). Quality of Life Research. doi:10.1007/s11136-016-1354-y.
Carlozzi, N. E., et al. (2016). The development of a new computer adaptive test to evaluate chorea in Huntington disease: HDQLIFE Chorea. Quality of Life Research.. doi:10.1007/s11136-016-1307-5.
Carlozzi, N. E., et al. (2016). HDQLIFE: the development of two new computer adaptive tests for use in Huntington disease, Speech Difficulties, and Swallowing Difficulties. Quality of Life Research. doi:10.1007/s11136-016-1273-y.
PROMIS® Instrument Development and Psychometric Evaluation Scientific Standards. http://www.nihpromis.org/Documents/PROMIS_Standards_050212.pdf.
Carlozzi, N. E., & Tulsky, D. S. (2013). Identification of health-related quality of life (HRQOL) issues relevant to individuals with Huntington disease. Journal of Health Psychology, 18(2), 212–225.
Kisala, P., & Tulsky, D. (2010). Opportunities for CAT applications in medical rehabilitation: Development of targeted item banks. Journal of Applied Measurement, 11(3), 315–330.
Tourangeau, R. (1984). Cognitive sciences and survey methods. In T. Jabine, et al. (Eds.), Cognitive Aspects of survey methodology: Building a bridge between disciplines (pp. 73–100). Washington, DC: National Academy Press.
MetaMetrics. (1995). The LEXILE framework for reading. Durham, NC: MetaMetrics Inc.
Shoulson, I., & Fahn, S. (1979). Huntington disease—Clinical care and evaluation. Neurology, 29(1), 1–3.
Hanauer, D. A., et al. (2015). Supporting information retrieval from electronic health records: A report of University of Michigan’s nine-year experience in developing and using the Electronic Medical Record Search Engine (EMERSE). Journal of Biomedical Informatics, 55, 290–300.
Paulsen, J. S., et al. (2006). Preparing for preventive clinical trials: The predict-HD study. Archives of Neurology, 63(6), 883–890.
Paulsen, J. S., et al. (2008). Detection of Huntington’s disease decades before diagnosis: The Predict-HD study. Journal of Neurology, Neurosurgery and Psychiatry, 79(8), 874–880.
Paulsen, J. S., et al. (2014). Clinical and biomarker changes in premanifest huntington disease show trial feasibility: A decade of the PREDICT-HD study. Frontiers in Aging Neuroscience, 6, 78.
Huntington Study Group. (1996). Unified Huntington’s Disease Rating Scale: Reliability and consistency. Movement Disorders, 11(2), 136–142.
Hogarth, P., et al. (2005). Interrater agreement in the assessment of motor manifestations of Huntington’s disease. Movement Disorders, 20(3), 293–297.
Huntington Study Group. (2006). Tetrabenazine as antichorea therapy in Huntington disease: A randomized controlled trial. Neurology, 66(3), 366–372.
Siesling, S., et al. (1998). Unified Huntington’s disease rating scale: A follow up. Movement Disorders, 13(6), 915–919.
Tabrizi, S. J., et al. (2011). Biological and clinical changes in premanifest and early stage Huntington’s disease in the TRACK-HD study: The 12-month longitudinal analysis. Lancet Neurology, 10(1), 31–42.
Busse, M., et al. (2011). Utilisation of Healthcare and Associated Services in Huntington’s disease: A data mining study. PLoS Currents, 3, RRN1206.
Carlozzi, N. E., et al. (2014). Understanding the outcomes measures used in huntington disease pharmacological trials: A systematic review. Journal of Huntington’s Disease, 3(3), 233–252.
National Institute on Neurological Disorders and Stroke: NINDS Common Data Elements. [cited May 23, 2011]. Available from: http://www.commondataelements.ninds.nih.gov/.
Smith, A. (1982). Symbol digit modalities test: Manual. Los Angeles: Western Psychological Services.
Stroop, J. R. (1992). Studies of interference in serial verbal reactions (Reprinted from Journal Experimental-Psychology, Vol. 18, pp. 643–662, 1935). Journal of Experimental Psychology-General, 121(1): 15–23.
Stroop, J. R. (1935). Studies of interference in serial verbal reactions. Journal of Experimental Psychology, 18, 643–662.
Craufurd, D., Thompson, J. C., & Snowden, J. S. (2001). Behavioral changes in Huntington disease. Neuropsychiatry, Neuropsychology, & Behavioral Neurology, 14(4), 219–226.
World Health Organization, W. (2012). The World Health Organization Disability Assessment Scale, WHODAS II. Available from: http://www.who.int/icidh/whodas/generalinfo.html.
Rabin, R., & de Charro, F. (2001). EQ-5D: A measure of health status from the EuroQol group. Annals of Medicine, 33(5), 337–343.
Lord, F. M. (1980). Applications of item response theory to practical testing problems. Hillside, NJ: Erlbaum.
De Ayala, R. J. (2009). The theory and practice of item response theory. New York: The Guilford Press.
PARSCALE. (2003). Scientific Software International Inc.: Lincolnwood, IL. http://www.ssicentral.com/irt/downloads.html.
Muthén, L. K., & Muthén, B. O. (2011). Mplus User’s Guide. Los Angeles, CA: Muthén & Muthén.
McDonald, R. P. (1999). Test theory: A unified treatment. Mahwah, NJ: Lawrence Erlbaum Associates Inc.
Cook, K. F., Kallen, M. A., & Amtmann, D. (2009). Having a fit: Impact of number of items and distribution of data on traditional criteria for assessing IRT’s unidimensionality assumption. Quality of Life Research, 18(4), 447–460.
Reise, S. P., Morizot, J., & Hays, R. D. (2007). The role of the bifactor model in resolving dimensionality issues in health outcomes measures. Quality of Life Research, 16(Suppl 1), 19–31.
Samejima, F., van der Liden, W. J., & Hambleton, R. (1996). The graded response model. In W. J. van der Liden (Ed.), Handbook of modern item response theory (pp. 85–100). NY: Springer.
Cai, L., Thissen, D., & du Toit, S. H. C. (2011). IRTPRO for windows [Computer software]. 2011, Lincolnwood, IL: Scientific Software International.
R Core Team. (2014). R: A language and environment for statistical computing. Vienna: R Foundation for Statistical Computing.
Choi, S. W., Gibbons, L. E., & Crane, P. K. (2011). Lordif: An R package for detecting differential item functioning using iterative hybrid ordinal logistic regression/item response theory and monte carlo simulations. Journal of Statistical Software, 39(8), 1–30.
Ustun, T. B., et al. (2010). Developing the World Health Organization Disability Assessment Schedule 2.0. Bulletin of the World Health Organization, 88(11), 815–823.
Cohen, J. (1988). Statistical power analysis for the behavioral sciences (2nd ed.). New York: Academic Press.
Samejima, F. (1969). Estimation of latent ability using a response pattern of graded scores (Psychometric Monograph No. 17). Richmond, VA: Psychometric Society.
Bryant, F. B., & Yarnold, P. R. (1995). Principal components analysis and exploratory and confirmatory factor analysis. In L. G. Grimm & R. R. Yarnold (Eds.), Reading and understanding multivariate statistics (pp. 99–136). Washington, DC: American Psychological Association.
Everitt, B. S. (1975). Multivariate analysis: The need for data, and other problems. British Journal of Psychiatry, 126, 237–240.
Gorsuch, R. L., & Analysis, Factor. (1983). Hillsdale. NJ: Lawrence Erlbaum Associates.
Clauser, B. E., & Hambleton, R. K. (1994). Review of differential item functioning, P. W. Holland, H. Wainer. Journal of Educational Measurement, 31(1), 88–92.
Anastasi, A., & Urbina, S. (1997). Psychological testing (7th ed.). Upper Saddle River, NJ: Prentice Hall.
Tabrizi, S. J., et al. (2012). Potential endpoints for clinical trials in premanifest and early Huntington’s disease in the TRACK-HD study: Analysis of 24 month observational data. Lancet Neurology, 11(1), 42–53.
Basch, E. (2010). The missing voice of patients in drug-safety reporting. New England Journal of Medicine, 362(10), 865–869.
Smith, K. M., & Dahodwala, N. (2014). Sex differences in Parkinson’s disease and other movement disorders. Experimental Neurology, 259, 44–56.
Tabrizi, S. J., et al. (2009). Biological and clinical manifestations of Huntington’s disease in the longitudinal TRACK-HD study: Cross-sectional analysis of baseline data. Lancet Neurology, 8(9), 791–801.
Paulsen, J. S., et al. (2014). Clinical and biomarker changes in premanifest Huntington disease show trial feasibility: A decade of the PREDICT-HD study. Front Aging Neurosci, 6, 78.
Dunn, K. M., et al. (2004). Patterns of consent in epidemiologic research: Evidence from over 25,000 responders. American Journal of Epidemiology, 159(11), 1087–1094.
Burg, J. A., Allred, S. L., & Sapp, J. H, 2nd. (1997). The potential for bias due to attrition in the National Exposure Registry: An examination of reasons for nonresponse, nonrespondent characteristics, and the response rate. Toxicology and Industrial Health, 13(1), 1–13.
Eagan, T. M., et al. (2002). Nonresponse in a community cohort study: Predictors and consequences for exposure-disease associations. Journal of Clinical Epidemiology, 55(8), 775–781.
Haga, S. B., et al. (2013). Public knowledge of and attitudes toward genetics and genetic testing. Genetic Testing and Molecular Biomarkers, 17(4), 327–335.
Roberts, J. S., et al. (2004). Who seeks genetic susceptibility testing for Alzheimer’s disease? Findings from a multisite, randomized clinical trial. Genetics in Medicine, 6(4), 197–203.
Huntington Study Group, P.I., et al. (2016). Clinical-genetic associations in the prospective Huntington at Risk Observational Study (PHAROS): Implications for clinical trials. JAMA Neurology, 73(1), 102–110.
Pringsheim, T., et al. (2012). The incidence and prevalence of Huntington’s disease: A systematic review and meta-analysis. Movement Disorders, 27(9), 1083–1091.
Folstein, S. E. (1989). Huntington’s disease: A disorder of families. Baltimore: Johns Hopkins University Press.
Hayden, M. R., MacGregor, J. M., & Beighton, P. H. (1980). The prevalence of Huntington’s chorea in South Africa. South African Medical Journal, 58, 193–196.
Narabayashi, H. (1973). Huntington’s chorea in Japan: Review of the literature. Advances in Neurology, 1, 253–259.
Duff, K., et al. (2010). Mild cognitive impairment in prediagnosed Huntington disease. Neurology, 75(6), 500–507.
Rutherford, C., et al. (2016). Mode of administration does not cause bias in patient-reported outcome results: A meta-analysis. Quality of Life Research, 25(3), 559–574.
Duff, K., et al. (2010). “Frontal” behaviors before the diagnosis of Huntington’s disease and their relationship to markers of disease progression: Evidence of early lack of awareness. Journal of Neuropsychiatry and Clinical Neurosciences, 22(2), 196–207.
Bull, M. T., et al. (2014). A pilot study of virtual visits in Huntington disease. Journal of Huntington’s Disease, 3(2), 189–195.
Acknowledgments
Work on this manuscript was supported by the National Institutes of Health (NIH), National Institute of Neurological Disorders and Stroke (R01NS077946), and the National Center for Advancing Translational Sciences (UL1TR000433). In addition, a portion of this study sample was collected in conjunction with the Predict-HD study. The Predict-HD study was supported by the NIH, National Institute of Neurological Disorders and Stroke (R01NS040068), the NIH, Center for Inherited Disease Research (provided supported for sample phenotyping), and the CHDI Foundation (award to the University of Iowa). We thank the University of Iowa, the Investigators and Coordinators of this study, the study participants, the National Research Roster for Huntington Disease Patients and Families, the Huntington Study Group, and the Huntington’s Disease Society of America. We acknowledge the assistance of Jeffrey D. Long, Hans J. Johnson, Jeremy H. Bockholt, Roland Zschiegner, and Jane S. Paulsen. We also acknowledge Roger Albin, Kelvin Chou, and Henry Paulsen for the assistance with participant recruitment. The content is solely the responsibility of the authors and does not necessarily represent the official views of the NIH.
HDQLIFE Site Investigators and Coordinators
Noelle Carlozzi, Praveen Dayalu, Stephen Schilling, Amy Austin, Matthew Canter, Siera Goodnight, Jennifer Miner, Nicholas Migliore (University of Michigan, Ann Arbor, MI); Jane Paulsen, Nancy Downing, Isabella DeSoriano, Courtney Shadrick, Amanda Miller (University of Iowa, Iowa City, IA); Kimberly Quaid, Melissa Wesson (Indiana University, Indianapolis, IN); Christopher Ross, Gregory Churchill, Mary Jane Ong (Johns Hopkins University, Baltimore, MD); Susan Perlman, Brian Clemente, Aaron Fisher, Gloria Obialisi, Michael Rosco (University of California Los Angeles, Los Angeles, CA); Michael McCormack, Humberto Marin, Allison Dicke (Rutgers University, Piscataway, NJ); Joel Perlmutter, Stacey Barton, Shineeka Smith (Washington University, St. Louis, MO); Martha Nance, Pat Ede (Struthers Parkinson’s Center); Stephen Rao, Anwar Ahmed, Michael Lengen, Lyla Mourany, Christine Reece, (Cleveland Clinic Foundation, Cleveland, OH); Michael Geschwind, Joseph Winer (University of California—San Francisco, San Francisco, CA), David Cella, Richard Gershon, Elizabeth Hahn, Jin-Shei Lai (Northwestern University, Chicago, IL).
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
N.E. Carlozzi currently has research grants from the NIH; she is also supported by grant funding from the NIH, NIDILRR, and CHDI; she declares no conflicts of interest; S.G. Schilling has a research grant from NSF. He also is supported by grant funding from NIH. He declares no conflicts of interest; J.-S. Lai currently has research grants from the NIH; she declares no conflicts of interest; J.S. Paulsen currently has research grants from the NIH; she is also supported by grant funding from NIH, NINDS, and CHDI; she declares no conflicts of interest; E.A. Hahn currently has research grants from the NIH; she is also supported by grant funding from the NIH and PCORI, and by research contracts from Merck and EMMES; she declares no conflicts of interest; J.S. Perlmutter currently has funding from the NIH, HDSA, CHDI, and APDA. He has received honoraria from the University of Rochester, American Academy of Neurology, Movement Disorders Society, Toronto Western Hospital, St. Luke’s Hospital in St. Louis, Emory University, Penn State University, Alberta innovates, Indiana Neurological Society, Parkinson Disease Foundation, Columbia University, St. Louis University, Harvard University and the University of Michigan; C.A. Ross declares no conflicts of interest; N.R. Downing declares no conflicts of interest; A.L. Kratz currently has research grants from the NIH and the Craig H. Neilsen Foundation; she is also supported by grant funding from the NMSS; she declares no conflicts of interest; M.K. McCormack currently has grants from the NJ Department of Health; he declares no conflicts of interest. M.A. Nance declares no conflicts of interest; K.A. Quaid has research funding from NIH, NIA, NCRR, NINDS and CHDI. She also has funding from HDSA. She has no conflicts of interest to declare; J.C. Stout has received research funding in the past three years from the Australian National Health and Medical Research Council, University College London, the CHDI Foundation, Prana Biotechnology, and the University of California, Davis. She is a Director of Stout Neuropsych Pty Ltd, which has received funding from Omeros, Teva Pharmaceuticals, Vaccinex, and Isis. She has been a consultant to Prana Biotechnology and Roche. She receives compensation as a member of the Board of the Huntington’s Study Group. R.C. Gershon receives research funds from numerous NIH institutes and the Department of Defense. He also receives consulting funds from AO Outcome Center, LLC (a for-profit arm of the nonprofit AO Foundation) and the American Board of Foot and Ankle Surgery; R.E. Ready declares that she has no conflicts of interest; J.A. Miner is supported by research grants from the NIH; she declares no conflict of interest; S.K. Barton is supported by grant funding from the Huntington’s Disease Society of America, CHDI Foundation and the NIH. She declares no conflicts of interest; S.L. Perlman is supported by grant funding from the NIH, CHDI, FARA, NAF, and several pharmaceutical companies (Edison, Horizon, Pfizer, Reata, Retrotope, Shire, Teva); she declares no conflicts of interest; S.M. Rao has received research grants from NIH, Department of Defense, National MS Society, CHDI Foundation and Biogen, and honoraria from the International Neuropsychological Society, Biogen and Genzyme; he declares no conflicts of interest; S. Frank receives salary support from the Huntington Study Group for a study sponsored by Auspex Pharmaceuticals. There is no conflict of interest; I. Shoulson has received research grants from the Food and Drug Administration (FDA), National Institutes of Health (NINDS, NHGRI) and the Parkinson’s Disease Foundation (NY, NY). He has also received speaker honoraria from the American Academy of Neurology and JAMA Neurology as an associate editor. Since May 2014, Dr. Shoulson has been a non-executive director of Prana Biotechnology Ltd (Melbourne, Australia), for which he is compensated for director and consulting services but has no equity positions or stock options in the company. He declares no conflicts of interest as a co-author of the submitted research report. H. Marin currently has grants from the NJ Department of Health; he declares no conflicts of interest. M.D. Geschwind currently has research grants from the NIH/NIA and Quest Diagnostics; he is also supported by grant funding from Cure PSP and Tau Consortium. He does consulting for MedaCorp, Inc, Gerson-Lehrman Group, Best Doctors, Advance Medical, Inc. and Optio, LLC. He receives compensation for multiple Grand Round lectures. He also gets funding for his research work from the Michael J Homer Family Fund. He discloses no conflicts of interest. P. Dayalu currently has research grants from the NIH, Astra-Zeneca, and Vaccinex. He declares no conflicts of interest. S.M. Goodnight is supported by grant funding from the NIH and the Craig H. Neilsen Foundation; she declares no conflicts of interest. D. Cella receives grant funding from the National Institutes of Health and reports that he has no conflicts of interest.
Ethical approval
All procedures performed in studies involving human participants were in accordance with the ethical standards of the institutional and/or national research committee and with the 1964 Declaration of Helsinki and its later amendments or comparable ethical standards.
Informed consent
Informed consent was obtained from all individual participants included in the study.
Rights and permissions
About this article
Cite this article
Carlozzi, N.E., Schilling, S.G., Lai, JS. et al. HDQLIFE: development and assessment of health-related quality of life in Huntington disease (HD). Qual Life Res 25, 2441–2455 (2016). https://doi.org/10.1007/s11136-016-1386-3
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11136-016-1386-3