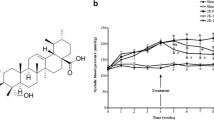
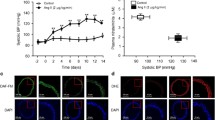
Abstract
Hypertension is a global health problem and leads to cardiovascular disease and renal injury. Solanum muricatum Aiton leaf extract, rich in flavonoids, is known for its antioxidant capacity. However, the effects of Solanum muricatum Aiton leaf extract on hypertension combined with inflammatory complications were unknown. This study aimed to investigate the impact of Solanum muricatum Aiton leaf extract on hypertension in vivo and in vitro. In vivo, Solanum muricatum Aiton leaf extract led to decrease high blood pressure, improve heart, aorta, and kidney pathology, and enhance the antioxidative activity in spontaneously hypertensive rats (SHR). Our study demonstrated Solanum muricatum Aiton leaf extract inhibited angiotensin-converting enzyme (ACE), epithelial sodium channel (ENaC), sodium glucose co-transporters-1 (SGLT-1), nuclear factor kappa B (NF-κB), cyclooxygenase-2 (COX-2), inducible nitric oxide synthase (iNOS), tumor necrosis factor-α (TNF-α), interleukin-1β (IL-1β), and interleukin-6 (IL-6). In vitro, Solanum muricatum Aiton leaf extract improved the angiotensin II-induced reactive oxygen species (ROS) and mitochondrial membrane depolarization in NRK-52E cells. Besides, Solanum muricatum Aiton leaf extract could also decrease the expressions of ENaC, SGLT-1, and NF-κB in angiotensin II-treated NRK-52E cells. Solanum muricatum Aiton leaf can be suggested as a novel antihypertensive agent ameliorating hypertension via ACE inhibition, inflammation reduction, and ROS. PLE is a novel anti-hypertensive agent to ameliorate hypertension and its complications, including inflammation.




Similar content being viewed by others
Data Availability
No datasets were generated or analysed during the current study.
References
Singh Y, Gupta G, Satija S, Negi P, Chellappan DK, Dua K (2020) RAAS blockers in hypertension posing a higher risk toward the COVID-19. Dermatol Ther 33(4):e13501. https://doi.org/10.1111/dth.13501
Atlas SA (2007) The renin-angiotensin aldosterone system: pathophysiological role and pharmacologic inhibition. J Manag Care Pharm 13:9–20. https://doi.org/10.18553/jmcp.2007.13.s8-b.9
Brem AS, Morris DJ, Gong R (2011) Aldosterone-induced fibrosis in the kidney: questions and controversies. Am J Kidney Dis 58:471–479. https://doi.org/10.1053/j.ajkd.2011.03.029
Lang F, Huang DY, Vallon V (2010) SGK, renal function and hypertension. J Nephrol 23:124–129. https://doi.org/10.1053/j.ajkd.2011.03.029
Marunaka Y, Niisato N, Taruno A et al (2011) Regulation of epithelial sodium transport via epithelial na + channel. J Biomed Biotechnol 2011:978196. https://doi.org/10.1155/2011/978196
Verrey F, Loffing J, Zecevic M, Heitzmann D, Staub O (2003) SGK1: aldosterone-induced relay of Na+ transport regulation in distal kidney nephron cells. Cell Physiol Biochem 13:21–28. https://doi.org/10.1159/000070246
Viedt C, Soto U, Krieger-Brauer HI et al (2000) Differential activation of mitogen-activated protein kinases in smooth muscle cells by angiotensin II: involvement of p22phox and reactive oxygen species. Arterioscler Thromb Vasc Biol 20:940–948. https://doi.org/10.1161/01.atv.20.4.940
Benigni A, Cassis P, Remuzzi G (2010) Angiotensin II revisited: new roles in inflammation, immunology and aging. EMBO Mol Med 2:247–257. https://doi.org/10.1002/emmm.201000080
Du X, Tao Q, Du H, Zhao Z, Dong Y, He S, Shao R, Wang Y, Han W, Wang X, Zhu Y (2021) Tengdan capsule prevents hypertensive kidney damage in SHR by inhibiting periostin-mediated renal fibrosis. Front Pharmacol 12:638298. https://doi.org/10.3389/fphar.2021.638298
Hsu JY, Lin HH, Chyau CC, Wang ZH, Chen JH (2021) Aqueous extract of pepino leaves ameliorates palmitic acid-induced hepatocellular lipotoxicity via Inhibition of endoplasmic reticulum stress and apoptosis. Antioxidants 10:903. https://doi.org/10.3390/antiox10060903
Hsu JY, Lin HH, Hsu CC, Chen BC, Chen JH (2018) Aqueous extract of Pepino (Solanum muriactum Ait) leaves ameliorate lipid accumulation and oxidative stress in alcoholic fatty liver disease. Nutrients 10:931. https://doi.org/10.3390/nu10070931
Masi S, Uliana M, Virdis A (2019) Angiotensin II and vascular damage in hypertension: role of oxidative stress and sympathetic activation. Vascul Pharmacol 115:13–17. https://doi.org/10.1016/j.vph.2019.01.004
Dandona P, Dhindsa S, Ghanim H, Chaudhuri A (2007) Angiotensin II and inflammation: the effect of angiotensin-converting enzyme inhibition and angiotensin II receptor blockade. J Hum Hypertens 21:20–27. https://doi.org/10.1038/sj.jhh.1002101
Dorresteijn JA, Visseren FL, Spiering W (2012) Mechanisms linking obesity to hypertension. Obes Rev 13:17–26. https://doi.org/10.1111/j.1467-789X.2011.00914.x
Lee IH, Campbell CR, Cook DI, Dinudom A (2008) Regulation of epithelial na + channels by aldosterone: role of Sgk1. Clin Exp Pharmacol Physiol 35:235–241. https://doi.org/10.1111/j.1440-1681.2007.04844.x
Marunaka Y (2017) Actions of quercetin, a flavonoid, on ion transporters: its physiological roles. Ann NY Acad Sci 1398:142–151. https://doi.org/10.1111/nyas.13361
Paredes MD, Romecín P, Atucha NM et al (2018) Beneficial effects of different flavonoids on vascular and renal function in L-NAME hypertensive rats. Nutrients 10:484. https://doi.org/10.3390/nu10040484
Veerappan R, Senthilkumar R (2015) Chrysin enhances antioxidants and oxidative stress in L-NAME-induced hypertensive rats. Int J Nutr Pharmacol Neurol Dis 5:20. https://doi.org/10.4103/2231-0738.150069
Cao Y, Xie L, Liu K et al (2021) The antihypertensive potential of flavonoids from Chinese herbal medicine: a review. Pharmacol Res 174:105919. https://doi.org/10.1016/j.phrs.2021.105919
Padilla E, Ruiz E, Redondo S, Gordillo-Moscoso A, Slowing K, Tejerina T (2005) Relationship between vasodilation capacity and phenolic content of Spanish wines. Eur J Pharmacol 517:84–91. https://doi.org/10.1016/j.ejphar.2005.04.044
de Oliveira MG, Nadruz W Jr, Mónica FZ (2022) Endothelial and vascular smooth muscle dysfunction in hypertension. Biochem Pharmacol 205:115263. https://doi.org/10.1016/j.bcp.2022.115263
Montezano AC, Touyz RM (2012) Molecular mechanisms of hypertension—reactive oxygen species and antioxidants: a basic science update for the clinician. Can J Cardiol 28:288–295. https://doi.org/10.1016/j.cjca.2012.01.017
Kuczeriszka M, Wąsowicz K (2022) Animal models of hypertension: the status of nitric oxide and oxidative stress and the role of the renal medulla. Nitric Oxide 125–126:40–46. https://doi.org/10.1016/j.niox.2022.06.003
Nair AB, Jacob S (2016) A simple practice guide for dose conversion between animals and human. J Basic Clin Pharm 7(2):27–31. https://doi.org/10.4103/0976-0105.177703
Funding
This work was supported by a grant from the National Science Council (NSC106-2320-B-040-008), Taiwan.
Author information
Authors and Affiliations
Contributions
H.-H.L.: Conceptualization, Methodology, Supervision, Writing—review and editing. C.-L.T.: Software, Visualization, Writing-Original draft. C.-Y.T.: Investigation, Visualization. P.-R.Y.: Investigation, Visualization. P.-Y.C.: Investigation, Data Curation, Writing-Original draft. C.-C.H.: Data Curation, Methodology. J.-H.C.: Conceptualization, Methodology, Supervision, Writing-Original draft, Project administration, Writing-review & editing, Funding acquisition.
Corresponding author
Ethics declarations
Ethical Approval
The animal experiment was approved by the Chung Shan Medical University Animal Care Committee (IACUC approval number: 1798).
Consent for Publication
All authors agreed to publish the results in Plant Foods for Human Nutrition.
Conflict of Interest
The authors declare no conflict of interest.
Competing Interests
The authors declare no competing interests.
Additional information
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Electronic Supplementary Material
Below is the link to the electronic supplementary material.
Rights and permissions
Springer Nature or its licensor (e.g. a society or other partner) holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Lin, HH., Tsai, CL., Tseng, CY. et al. Anti-Hypertensive Effect of Solanum muricatum Aiton Leaf Extract In Vivo and In Vitro. Plant Foods Hum Nutr 79, 182–188 (2024). https://doi.org/10.1007/s11130-024-01146-1
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11130-024-01146-1