Abstract
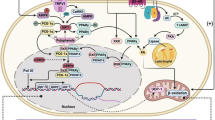
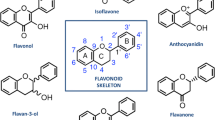
Obesity is a major global public health concern, limiting socio-economic development and human productivity. As studies focus on finding sustainable solutions to this challenge, polyphenols have shown promising results and have become a research focus. This is mainly because of associated lower risks of side effects with their use, compared to synthetic pharmaceuticals. In this study, the anti-obesity potentials of dietary polyphenols have been reviewed. Using a narrative approach, the biological activities of polyphenols and their influence on energy metabolism and mechanisms are discussed. Specifically, their roles in insulin-dependent glucose uptake, insulin sensitivity, lipid metabolism and storage in adipocytes, starch digestibility, and regulation of mitophagy and mitogenesis in muscle cells and adipocytes, were considered. After considering the major findings of many related studies, it was confirmed that polyphenols can prevent and ameliorate obesity by fighting insulin resistance (IR) induced by pro-inflammatory cytokines, scavenging reactive oxygen species (ROS) and limiting their effects, and by regulating the expression and/or activity of key enzymes along relevant pathways. More human studies are needed to reveal more about the anti-obesity effects of dietary polyphenols and their effective doses in humans.




Similar content being viewed by others
Data Availability
Data sharing not applicable to this article as no datasets were generated or analysed during the current study.
Abbreviations
- IR:
-
Insulin resistance
- ROS:
-
Reactive oxygen species
- EGCG:
-
Epigallocatechin-3-O-gallate
- RSV:
-
Resveratrol
- GA:
-
Gallic acid
- CRC:
-
Curcumin
- IS:
-
Insulin sensitivity
- CR:
-
Caloric restriction
- GLUT:
-
Glucose transporter
- GSV:
-
Glucose transporter storage vesicle
- NF-kB:
-
Nuclear factor kappa B
- JNK:
-
C-Jun N-terminal kinase
- SFA:
-
Saturated fatty acid
- TNF α:
-
Tumor necrosis factor alpha
- NADPH:
-
Reduced nicotinamide adenine dinucleotide phosphate
- NOX:
-
NADPH oxidase
- IRS-1:
-
Insulin receptor substrate 1
- PI3K:
-
Phosphatidylinositol-3-kinase
- Akt:
-
Collective name of a set of three serine/threonine-specific protein kinases
- NO:
-
Nitric oxide
- eNOS:
-
Endothelial NO synthase
- mTOR:
-
Mammalian target of rapamycin
- HepG2:
-
Human liver cells
- IR-HepG2:
-
Insulin resistant human liver cells
- PEPCK:
-
Phosphoenolpyruvate carboxykinase
- G6Pase:
-
Glucose-6-phosphatase
- LDL:
-
Low density lipoprotein
- HDL:
-
High density lipoprotein
- RGPD:
-
Red grape pomace drink
- AMPK:
-
AMP-activated protein kinase
- PTB1B:
-
Protein tyrosine phosphatase 1B
- LKB1:
-
Liver kinase B1
- CaMKK:
-
Calcium/calmodulin-dependent protein kinase kinase
- RA:
-
Rosmarinic acid
- IGF-1:
-
Insulin-like growth factor 1
- SREBP:
-
Sterol regulatory-element binding proteins
- PPAR:
-
Peroxisome proliferator-activated receptors
- G3P:
-
Glyceraldehyde 3-phosphate
- GPDH:
-
Glycerol-3-phosphate dehydrogenase
- HMG-CoA:
-
3-Hydroxy-3-methylglutaryl coenzyme A
- FABP4:
-
Fatty acid binding protein 4
- LXR-α:
-
Liver X receptor alpha
- PGC-1:
-
Peroxisome proliferator-activated receptor-gamma coactivator-1alpha
- Ucpl:
-
Uncoupling protein 1
- CIDEA:
-
Cell death-inducing DNA fragmentation factor alpha-like effector A
- Tbx1:
-
Tata-box protein 1
- Cd137:
-
Tumor necrosis factor receptor superfamily member 9
- BAT:
-
Beige adipose tissue
- iWAT:
-
Inguinal white adipose tissues
- SIRT1:
-
Sirtuin 1
- SCD 1:
-
Stearoyl-Coenzyme A desaturase 1
- ATP:
-
Adenosine triphosphate
- NAD:
-
Nicotinamide adenine dinucleotide
- FOXO3:
-
Forkhead box O3
- UNC51:
-
Autophagy-related serine/threonine kinase
- ULK1:
-
UNC-51-like kinase 1
- PINK 1:
-
PTEN induced putative kinase 1
- PTEN:
-
A tumor suppressor phosphatase and tensin homolog
- ERK2:
-
Mitogen-activated protein kinase 1 (MAPK1)
- NAMPT:
-
Nicotinamide phosphoribosyltransferase
- PKD:
-
Protein kinase D
References
Fryar CD, Carroll MD, Afful J (2020) Prevalence of overweight, obesity, and severe obesity among adults aged 20 and over: United States, 1960–1962 through 2017–2018. Health E-Stats. NCHS Publications and Information Products, Atlanta, pp 1–7
Krzysztoszek J, Laudanska-Krzeminska I, Bronikowski M (2019) Assessment of epidemiological obesity among adults in EU countries. Ann Agric Environ Med 26(2):341–349. https://doi.org/10.26444/aaem/97226
Price AJ, Crampin AC, Amberbir A, Kayuni-Chihana N, Musicha C, Tafatatha T, Branson K, Lawlor DA, Mwaiyeghele E, Nkhwazi L, Smeeth L (2018) Prevalence of obesity, hypertension, and diabetes, and cascade of care in sub-Saharan Africa: a cross-sectional, population-based study in rural and urban Malawi. Lancet Diabetes Endocrinol 6(3):208–222. https://doi.org/10.1016/S2213-8587(17)30432-1
Hojjat TA (2021) Economics of obesity: poverty, income inequality, and health, 2nd edn. Springer Cham, Switzerland, pp 1–16. https://doi.org/10.1007/978-3-030-78487-4
Corrêa TA, Rogero MM (2019) Polyphenols regulating microRNAs and inflammation biomarkers in obesity. Nutrients 59:150–157. https://doi.org/10.1016/j.nut.2018.08.010
Hughes LA, Arts IC, Ambergen T, Brants HA, Dagnelie PC, Goldbohm RA, van den Brant PA, Weijenberg MP (2008) Higher dietary flavone, flavonol, and catechin intakes are associated with less of an increase in BMI over time in women: a longitudinal analysis from the Netherlands Cohort Study. Am J Clin Nutr 88(5):1341–1352. https://doi.org/10.3945/ajcn.2008.26058
Grosso G, Stepaniak U, Micek A, Topor-Mądry R, Pikhart H, Szafraniec K, Pająk A (2015) Association of daily coffee and tea consumption and metabolic syndrome: results from the Polish arm of the HAPIEE study. Eur J Nutr 54(7):1129–1137. https://doi.org/10.1007/s00394-014-0789-6
Tanabe H, Pervin M, Goto S, Isemura M, Nakamura Y (2017) Beneficial effects of plant polyphenols on obesity. Obes Control Ther 4:1–16. https://doi.org/10.15226/2374-8354/4/3/00142
Ohishi T, Fukutomi R, Shoji Y, Goto S, Isemura M (2021) The beneficial effects of principal polyphenols from green tea, coffee, wine, and curry on obesity. Molecules 26(2):453. https://doi.org/10.3390/2Fmolecules26020453
Suzuki T, Pervin M, Goto S, Isemura M, Nakamura Y (2016) Beneficial effects of tea and the green tea catechin epigallocatechin-3-gallate on obesity. Molecules 21(10):1305. https://doi.org/10.3390/molecules21101305
Wang P, Li D, Ke W, Liang D, Hu X, Chen F (2020) Resveratrol-induced gut microbiota reduces obesity in high-fat diet-fed mice. Int J Obes 44(1):213–225. https://doi.org/10.1038/s41366-019-0332-1
Giovinazzo G, Grieco F (2015) Functional properties of grape and wine polyphenols. Plant Foods Hum Nutr 70(4):454–462. https://doi.org/10.1007/s11130-015-0518-1
Rocchetti G, Giuberti G, Gallo A, Bernardi J, Marocco A, Lucini L (2018) Effect of dietary polyphenols on the in vitro starch digestibility of pigmented maize varieties under cooking conditions. Food Res Int 108:183–191
Cipolla-Neto J, Amaral F, Afeche S, Tan D, Reiter R (2014) Melatonin, energy metabolism, and obesity: a review. J Pineal Res 56(4):371–381. https://doi.org/10.1111/jpi.12137
Ramírez-Venegas G, Ita-Pérez D, Díaz-Muñoz M, Méndez I, García-Gasca T, Ahumada-Solórzano M, Zambrano-Estrada X, Vázquez-Martínez O, Guzmán-Maldonado H, Luna-Moreno D (2021) Supplementation with Phaseolus vulgaris leaves improves metabolic alterations induced by high-fat/fructose diet in rats under time-restricted feeding. Plant Foods Hum Nutr 76(3):297–303. https://doi.org/10.1007/s11130-021-00904-9
Satoh T (2014) Molecular mechanisms for the regulation of insulin-stimulated glucose uptake by small guanosine triphosphatases in skeletal muscle and adipocytes. Int J Mol Sci 15(10):18677–18692. https://doi.org/10.3390/ijms151018677
Vanhaesebroeck B, Stephens L, Hawkins P (2012) PI3K signalling: the path to discovery and understanding. Nat Rev Mol Cell Biol 13(3):195–203. https://doi.org/10.1038/nrm3290
Furtado LM, Somwar R, Sweeney G, Niu W, Klip A (2002) Activation of the glucose transporter GLUT4 by insulin. Biochem Cell Biol 80(5):569–578. https://doi.org/10.1139/o02-156
Wei Y, Chen K, Whaley-Connell AT, Stump CS, Ibdah JA, Sowers JR (2008) Skeletal muscle insulin resistance: role of inflammatory cytokines and reactive oxygen species. Am J Physiol Regul Integr Comp Physiol 294(3):R673–R680. https://doi.org/10.1152/ajpregu.00561.2007
Williamson G, Sheedy K (2020) Effects of polyphenols on insulin resistance. Nutrients 12(10):3135. https://doi.org/10.3390/nu12103135
Vilahur G, Padró T, Casaní L, Mendieta G, López JA, Streitenberger S, Badimon L (2015) Polyphenol-enriched diet prevents coronary endothelial dysfunction by activating the Akt/eNOS pathway. Rev Esp Cardiol 68(3):216–225. https://doi.org/10.1016/j.rec.2014.04.021
Soto-Covasich J, Reyes-Farias M, Torres RF, Vásquez K, Duarte L, Quezada J, Jimenez P, Pino MT, Garcia-Nannig L, Mercado L, Garcia-Diaz DF (2020) A polyphenol-rich Calafate (Berberis microphylla) extract rescues glucose tolerance in mice fed with cafeteria diet. J Funct Foods 67:103856. https://doi.org/10.1016/j.jff.2020.103856
Xia T, Duan W, Zhang Z, Fang B, Zhang B, Xu B, de la Cruz CBV, El-Seedi H, Simal-Gandara J, Wang S, Wang M (2021) Polyphenol-rich extract of Zhenjiang aromatic vinegar ameliorates high glucose-induced insulin resistance by regulating JNK-IRS-1 and PI3K/Akt signaling pathways. Food Chem 335:127513. https://doi.org/10.1016/j.foodchem.2020.127513
Costabile G, Vitale M, Luongo D, Naviglio D, Vetrani C, Ciciola P, Tura A, Castello F, Mena P, Del Rio D, Capaldo B (2019) Grape pomace polyphenols improve insulin response to a standard meal in healthy individuals: a pilot study. Clin Nutr 38(6):2727–2734. https://doi.org/10.1016/j.clnu.2018.11.028
Vlavcheski F, Naimi M, Murphy B, Hudlicky T, Tsiani E (2017) Rosmarinic acid, a rosemary extract polyphenol, increases skeletal muscle cell glucose uptake and activates AMPK. Molecules 22(10):1669. https://doi.org/10.3390/2Fmolecules22101669
Tsuda S, Egawa T, Ma X, Oshima R, Kurogi E, Hayashi T (2012) Coffee polyphenol caffeic acid but not chlorogenic acid increases 5′ AMP-activated protein kinase and insulin-independent glucose transport in rat skeletal muscle. J Nutr Biochem 23(11):1403–1409. https://doi.org/10.1016/j.jnutbio.2011.09.001
Lee S-H, Jeon Y-J (2013) Anti-diabetic effects of brown algae derived phlorotannins, marine polyphenols through diverse mechanisms. Fitoterapia 86:129–136. https://doi.org/10.1016/j.fitote.2013.02.013
Kk IM, Issac A, Ninan E, Kuttan R, Maliakel B (2014) Enhanced anti-diabetic activity of polyphenol-rich de-coumarinated extracts of Cinnamomum cassia. J Funct Foods 10:54–64. https://doi.org/10.1016/j.jff.2014.05.008
Hwang J-T, Kwon DY, Yoon SH (2009) AMP-activated protein kinase: a potential target for the diseases prevention by natural occurring polyphenols. N Biotechnol 26(1–2):17–22. https://doi.org/10.1016/j.nbt.2009.03.005
Matacchione G, Gurău F, Baldoni S, Prattichizzo F, Silvestrini A, Giuliani A, Pugnaloni A, Espinosa E, Amenta F, Bonafè M, Procopio AD (2020) Pleiotropic effects of polyphenols on glucose and lipid metabolism: focus on clinical trials. Ageing Res Rev 61:101074. https://doi.org/10.1016/j.arr.2020.101074
Chung E, Campise SN, Joiner HE, Tomison MD, Kaur G, Dufour JM, Cole L, Ramalingam L, Moustaid-Moussa N, Shen CL (2019) Effect of annatto-extracted tocotrienols and green tea polyphenols on glucose homeostasis and skeletal muscle metabolism in obese male mice. J Nutr Biochem 67:36–43. https://doi.org/10.1016/j.jnutbio.2019.01.021
Plaza M, Batista ÂG, Cazarin CBB, Sandahl M, Turner C, Östman E, Júnior MRM (2016) Characterization of antioxidant polyphenols from Myrciaria jaboticaba peel and their effects on glucose metabolism and antioxidant status: A pilot clinical study. Food Chem 211:185–197. https://doi.org/10.1016/j.foodchem.2016.04.142
Lee JH, Lee SY, Kim B, Seo WD, Jia Y, Wu C, Jun HJ, Lee SJ (2015) Barley sprout extract containing policosanols and polyphenols regulate AMPK, SREBP2 and ACAT2 activity and cholesterol and glucose metabolism in vitro and in vivo. Food Res Int 72:174–183. https://doi.org/10.1016/j.foodres.2015.03.041
Cheng DM, Pogrebnyak N, Kuhn P, Poulev A, Waterman C, Rojas-Silva P, Johnson WD, Raskin I (2014) Polyphenol-rich Rutgers Scarlet Lettuce improves glucose metabolism and liver lipid accumulation in diet-induced obese C57BL/6 mice. Nutrition 30(7–8):S52–S58. https://doi.org/10.1016/j.nut.2014.02.022
Li D, Yang Y, Sun L, Fang Z, Chen L, Zhao P, Wang Z, Guo Y (2020) Effect of young apple (Malus domestica Borkh. cv. Red Fuji) polyphenols on alleviating insulin resistance. Food Biosci 36:100637. https://doi.org/10.1016/j.fbio.2020.100637
Mokashi P, Khanna A, Pandita N (2017) Flavonoids from Enicostema littorale blume enhances glucose uptake of cells in insulin resistant human liver cancer (HepG2) cell line via IRS-1/PI3K/Akt pathway. Biomed Pharmacother 90:268–277. https://doi.org/10.1016/j.biopha.2017.03.047
Ooi DJ, Azmi NH, Imam MU, Alitheen NB, Ismail M (2018) Curculigoside and polyphenol-rich ethyl acetate fraction of Molineria latifolia rhizome improved glucose uptake via potential mTOR/AKT activated GLUT4 translocation. J Food Drug Anal 26(4):1253–1264. https://doi.org/10.1016/j.jfda.2018.03.003
Zygmunt K, Faubert B, MacNeil J, Tsiani E (2010) Naringenin, a citrus flavonoid, increases muscle cell glucose uptake via AMPK. Biochem Biophys Res Commun 398(2):178–183. https://doi.org/10.1016/j.bbrc.2010.06.048
Ma J, Zheng Y, Tang W, Yan W, Nie H, Fang J, Liu G (2020) Dietary polyphenols in lipid metabolism: a role of gut microbiome. Anim Nutr 6(4):404–409. https://doi.org/10.1016/j.aninu.2020.08.002
Les F, Arbonés-Mainar JM, Valero MS, López V (2018) Pomegranate polyphenols and urolithin A inhibit α-glucosidase, dipeptidyl peptidase-4, lipase, triglyceride accumulation and adipogenesis related genes in 3T3-L1 adipocyte-like cells. J Ethnopharmacol 220:67–74. https://doi.org/10.1016/j.jep.2018.03.029
Kim J, Kundu M, Viollet B, Guan K-L (2011) AMPK and mTOR regulate autophagy through direct phosphorylation of Ulk1. Nat Cell Biol 13(2):132–141. https://doi.org/10.1038/ncb2152
Cantó C, Gerhart-Hines Z, Feige JN, Lagouge M, Noriega L, Milne JC, Elliott PJ, Puigserver P, Auwerx J (2009) AMPK regulates energy expenditure by modulating NAD+ metabolism and SIRT1 activity. Nature 458(7241):1056–1060. https://doi.org/10.1038/nature07813
Rupasinghe HV, Sekhon-Loodu S, Mantso T, Panayiotidis MI (2016) Phytochemicals in regulating fatty acid β-oxidation: potential underlying mechanisms and their involvement in obesity and weight loss. Pharmacol Ther 165:153–163. https://doi.org/10.1016/j.pharmthera.2016.06.005
Tamura Y, Tomiya S, Takegaki J, Kouzaki K, Tsutaki A, Nakazato K (2020) Apple polyphenols induce browning of white adipose tissue. J Nutr Biochem 77:108299. https://doi.org/10.1016/j.jnutbio.2019.108299
Ali F, Ismail A, Esa NM, Pei CP, Kersten S (2015) Hepatic genome-wide expression of lipid metabolism in diet-induced obesity rats treated with cocoa polyphenols. J Funct Foods 17:969–978. https://doi.org/10.1016/j.jff.2015.06.047
Yoshino M, Haneda M, Naruse M, Htay HH, Iwata S, Tsubouchi R, Murakami K (2002) Prooxidant action of gallic acid compounds: copper-dependent strand breaks and the formation of 8-hydroxy-2′-deoxyguanosine in DNA. Toxicol In Vitro 16(6):705–709. https://doi.org/10.1016/S0887-2333(02)00061-9
Zou T, Chen D, Yang Q, Wang B, Zhu MJ, Nathanielsz PW, Du M (2017) Resveratrol supplementation of high-fat diet-fed pregnant mice promotes brown and beige adipocyte development and prevents obesity in male offspring. J Physiol 595(5):1547–1562. https://doi.org/10.1113/jp273478
Han X, Guo J, You Y, Yin M, Liang J, Ren C, Zhan J, Huang W (2018) Vanillic acid activates thermogenesis in brown and white adipose tissue. Food Funct 9(8):4366–4375. https://doi.org/10.1039/c8fo00978c
Chou Y-C, Ho C-T, Pan M-H (2018) Immature Citrus reticulata extract promotes browning of beige adipocytes in high-fat diet-induced C57BL/6 mice. J Agric Food Chem 66(37):9697–9703. https://doi.org/10.1021/acs.jafc.8b02719
Anhê FF, Choi B, Dyck J, Schertzer J, Marette A (2019) Host–microbe interplay in the cardiometabolic benefits of dietary polyphenols. Trends Endocrinol Metab 30(6):384–395. https://doi.org/10.1016/j.tem.2019.04.002
Aires V, Labbé J, Deckert V, Pais de Barros JP, Boidot R, Haumont M, Maquart G, Le Guern N, Masson D, Prost-Camus E, Prost M (2019) Healthy adiposity and extended lifespan in obese mice fed a diet supplemented with a polyphenol-rich plant extract. Sci Rep 9(1):1–16. https://doi.org/10.1038/2Fs41598-019-45600-6
Ishida N, Iizuka M, Kataoka K, Okazaki M, Shiraishi K, Yagi Y, Jobu K, Yokota J, Oishi M, Moriyama H, Shimamura T (2018) Improvement of blood lipid profiles by Goishi tea polyphenols in a randomised, double-blind, placebo-controlled clinical study. Int J Food Sci Nutr 69(5):598–607. https://doi.org/10.1080/09637486.2017.1386629
Dai J, Liang K, Zhao S, Jia W, Liu Y, Wu H, Lv J, Cao C, Chen T, Zhuang S, Hou X (2018) Chemoproteomics reveals baicalin activates hepatic CPT1 to ameliorate diet-induced obesity and hepatic steatosis. Proc Natl Acad Sci 115(26):E5896–E5905. https://doi.org/10.1073/2Fpnas.1801745115
Liou C-J, Wei C-H, Chen Y-L, Cheng C-Y, Wang C-L, Huang W-C (2018) Fisetin protects against hepatic steatosis through regulation of the Sirt1/AMPK and fatty acid β-oxidation signaling pathway in high-fat diet-induced obese mice. Cell Physiol Biochem 49(5):1870–1884. https://doi.org/10.1159/000493650
Ibitoye OB, Ajiboye TO (2018) Dietary phenolic acids reverse insulin resistance, hyperglycaemia, dyslipidaemia, inflammation and oxidative stress in high-fructose diet-induced metabolic syndrome rats. Arch Physiol Biochem 124(5):410–417. https://doi.org/10.1080/13813455.2017.1415938
Naowaboot J, Piyabhan P, Munkong N, Parklak W, Pannangpetch P (2016) Ferulic acid improves lipid and glucose homeostasis in high-fat diet-induced obese mice. Clin Exp Pharmacol Physiol 43(2):242–250. https://doi.org/10.1111/1440-1681.12514
Liu L, Yasen M, Tang D, Ye J, Aisa HA, Xin X (2018) Polyphenol-enriched extract of Rosa rugosa Thunb regulates lipid metabolism in diabetic rats by activation of AMPK pathway. Biomed Pharmacother 100:29–35. https://doi.org/10.1016/j.biopha.2018.01.143
Tenore GC, Calabrese G, Stiuso P, Ritieni A, Giannetti D, Novellino E (2014) Effects of Annurca apple polyphenols on lipid metabolism in HepG2 cell lines: a source of nutraceuticals potentially indicated for the metabolic syndrome. Food Res Int 63:252–257. https://doi.org/10.1016/j.foodres.2014.05.024
Sugiyama H, Akazome Y, Shoji T, Yamaguchi A, Yasue M, Kanda T, Ohtake Y (2007) Oligomeric procyanidins in apple polyphenol are main active components for inhibition of pancreatic lipase and triglyceride absorption. J Agric Food Chem 55(11):4604–4609. https://doi.org/10.1021/jf070569k
Tenore GC, Campiglia P, Stiuso P, Ritieni A, Novellino E (2013) Nutraceutical potential of polyphenolic fractions from Annurca apple (M. pumila Miller cv Annurca). Food Chem 140(4):614–622. https://doi.org/10.1016/j.foodchem.2012.10.112
Bahadoran Z, Mirmiran P, Azizi F (2013) Dietary polyphenols as potential nutraceuticals in management of diabetes: a review. J Diabetes Metab Disord 12(1):43. https://doi.org/10.1186/2F2251-6581-12-43
Palikaras K, Tavernarakis N (2014) Mitochondrial homeostasis: the interplay between mitophagy and mitochondrial biogenesis. Exp Gerontol 56:182–188. https://doi.org/10.1016/j.exger.2014.01.021
Haas RH (2019) Mitochondrial dysfunction in aging and diseases of aging. Biology 8(2):48. https://doi.org/10.3390/2Fbiology8020048
Chappell NR, Zhou B, Hosseinzadeh P, Schutt A, Gibbons WE, Blesson CS (2021) Hyperandrogenemia alters mitochondrial structure and function in the oocytes of obese mouse with polycystic ovary syndrome. Fertil Steril Sci 2(1):101–112. https://doi.org/10.1016/j.xfss.2020.12.001
Caldwell CC, Petzinger GM, Jakowec MW, Cadenas E (2020) Treadmill exercise rescues mitochondrial function and motor behavior in the CAG140 knock-in mouse model of Huntington’s disease. Chem Biol Interact 315:108907. https://doi.org/10.1016/j.cbi.2019.108907
Waldman M, Cohen K, Yadin D, Nudelman V, Gorfil D, Laniado-Schwartzman M, Kornwoski R, Aravot D, Abraham NG, Arad M, Hochhauser E (2018) Regulation of diabetic cardiomyopathy by caloric restriction is mediated by intracellular signaling pathways involving ‘SIRT1 and PGC-1α.’ Cardiovasc Diabetol 17(1):1–12. https://doi.org/10.1186/s12933-018-0754-4
Davinelli S, De Stefani D, De Vivo I, Scapagnini G (2020) Polyphenols as caloric restriction mimetics regulating mitochondrial biogenesis and mitophagy. Trends Endocrinol Metab 31(7):536–550. https://doi.org/10.1016/j.tem.2020.02.011
Das S, Mitrovsky G, Vasanthi HR, Das DK (2014) Antiaging properties of a grape-derived antioxidant are regulated by mitochondrial balance of fusion and fission leading to mitophagy triggered by a signaling network of Sirt1-Sirt3-Foxo3-PINK1-PARKIN. Oxid Med Cell Longev 2014:345105. https://doi.org/10.1155/2F2014/2F345105
Rocchetti G, Giuberti G, Busconi M, Marocco A, Trevisan M, Lucini L (2020) Pigmented sorghum polyphenols as potential inhibitors of starch digestibility: an in vitro study combining starch digestion and untargeted metabolomics. Food Chem 312:126077. https://doi.org/10.1016/j.foodchem.2019.126077
Yu X, Xu Y, Zhang S, Sun J, Liu P, Xiao L, Tang Y, Liu L, Yao P (2016) Quercetin attenuates chronic ethanol-induced hepatic mitochondrial damage through enhanced mitophagy. Nutrients 8(1):27. https://doi.org/10.3390/nu8010027
Chung S, Yao H, Caito S, Hwang JW, Arunachalam G, Rahman I (2010) Regulation of SIRT1 in cellular functions: role of polyphenols. Arch Biochem Biophys 501(1):79–90. https://doi.org/10.1016/j.abb.2010.05.003
Caito S, Rajendrasozhan S, Cook S, Chung S, Yao H, Friedman AE, Brookes PS, Rahman I (2010) SIRT1 is a redox-sensitive deacetylase that is post-translationally modified by oxidants and carbonyl stress. FASEB J 24(9):3145–3159. https://doi.org/10.1096/2Ffj.09-151308
Sun L, Miao M (2020) Dietary polyphenols modulate starch digestion and glycaemic level: a review. Crit Rev Food Sci Nutr 60(4):541–555. https://doi.org/10.1080/10408398.2018.1544883
Kan L, Oliviero T, Verkerk R, Fogliano V, Capuano E (2020) Interaction of bread and berry polyphenols affects starch digestibility and polyphenols bio-accessibility. J Funct Foods 68:103924. https://doi.org/10.1016/j.jff.2020.103924
Zhao B, Wang B, Zheng B, Chen L, Guo Z (2019) Effects and mechanism of high-pressure homogenization on the characterization and digestion behavior of lotus seed starch–green tea polyphenol complexes. J Funct Foods 57:173–181. https://doi.org/10.1016/j.jff.2019.04.016
Author information
Authors and Affiliations
Contributions
Courage Sedem Dzah conceptualized the study, drafted some sections, did the editing of the manuscript and finalized the paper. Emmanuel Letsyo and John Dzikunoo also drafted some sections of the paper and helped with proofreading, David Asante-Donyinah and Zeenatu Sugloh Adams contributed by drafting some sections of the paper. All authors read and approved the final version of the manuscript for submission.
Corresponding author
Ethics declarations
Competing Interests
The authors declare no competing interests.
Ethics Approval
Ethics approval not applicable as no human tissues nor human subjects were used during the current study. This study only reviewed available literature.
Consent to Participate
Not applicable as no human participants were used in the current study.
Consent for Publication
All authors have agreed to publish this paper without any reservations whatsoever.
Conflicts of Interest Statement
The authors have no competing interests to declare that are relevant to the content of this article.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Springer Nature or its licensor (e.g. a society or other partner) holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Dzah, C.S., Asante-Donyinah, D., Letsyo, E. et al. Dietary Polyphenols and Obesity: A Review of Polyphenol Effects on Lipid and Glucose Metabolism, Mitochondrial Homeostasis, and Starch Digestibility and Absorption. Plant Foods Hum Nutr 78, 1–12 (2023). https://doi.org/10.1007/s11130-022-01034-6
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11130-022-01034-6