Abstract
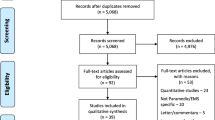
Background: Post-traumatic stress disorder (PTSD) has debilitating effects on quality of life. Patient-reported outcomes (PROs) assess changes in quality of life and serve as subjective measurements of patient experience. The aim of this study is to assess the completeness of PRO reporting within randomized controlled trials with interventions pertaining to PTSD. Methods: This cross-sectional, meta-epidemiological study assessed the completeness of PRO reporting in RCTs investigating PTSD interventions. We searched multiple databases for published RCTs of PTSD interventions that used PROs as a primary or secondary outcome. We assessed PRO completeness using the PRO adaptation of the Consolidated Standards of Reporting Trial (CONSORT). We used a bivariate regression model to determine the association between trial characteristics and the completeness of reporting. Results: After an initial screening of 5906 articles, our final sample of RCTs for inclusion was 43. The mean completeness of reporting of PROs was 58.4% (SD = 14.50). We found no significant associations between trial characteristics and completeness of the CONSORT-PRO adaptation. Conclusion: Reporting of PROs was often incomplete among RCTs focused on PTSD. We believe that adherence to CONSORT-PRO will improve both PRO reporting and implementation into clinical practice to improve assessment of quality of life.

Similar content being viewed by others
Data and Material Availability
References
“Psychiatry.org -. What is Posttraumatic Stress Disorder (PTSD)?” https://www.psychiatry.org/patients-families/ptsd/what-is-ptsd (accessed Oct.17, 2022).
“What Is PTSD?,” American Psychiatric Association. https://www.psychiatry.org/patients-families/ptsd/what-is-ptsd (accessed Jun.29, 2021).
Bedi US, Arora R. “Cardiovascular manifestations of posttraumatic stress disorder,” J. Natl. Med. Assoc., vol. 99, no. 6, pp. 642–649, Jun. 2007.
Gradus JL, Farkas DK, Svensson E, Ehrenstein V, Lash TL, Toft H, Sørensen. Posttraumatic stress disorder and gastrointestinal Disorders in the danish Population. Epidemiology. May 2017;28(3):354–60.
Fox V, Dalman C, Dal H, Hollander A-C, Kirkbride JB, Pitman A. Suicide risk in people with post-traumatic stress disorder: a cohort study of 3.1 million people in Sweden. J Affect Disord. Jan. 2021;279:609–16.
Rivera SC, Kyte DG, Aiyegbusi OL, Slade AL, McMullan C, Calvert MJ. “The impact of patient-reported outcome (PRO) data from clinical trials: a systematic review and critical analysis,” Health Qual. Life Outcomes, vol. 17, no. 1, p. 156, Oct. 2019.
Drug DPID. “Guidance for industry,” Center for Drug Evaluation and Research (CDER), vol. 1000, 1998, [Online]. Available: https://www.fda.gov/media/77832/download
Mercieca-Bebber R, King MT, Calvert MJ, Stockler MR, Friedlander M. “The importance of patient-reported outcomes in clinical trials and strategies for future optimization,” Patient Relat. Outcome Meas., vol. 9, pp. 353–367, Nov. 2018.
García-Elorrio E, Aziz S. The need for standardized reporting of research findings in the field of quality of care. Int J Qual Health Care. Mar. 2021;33(1). https://doi.org/10.1093/intqhc/mzab040.
Schulz KF, Altman DG, Moher D, Group CONSORT. “CONSORT 2010 statement: updated guidelines for reporting parallel group randomised trials,” BMJ, vol. 340, p. c332, Mar. 2010.
Calvert M et al. “Reporting of patient-reported outcomes in randomized trials: the CONSORT PRO extension,” JAMA, vol. 309, no. 8, pp. 814–822, Feb. 2013.
Friedlander M, Mercieca-Bebber RL, King MT. Patient-reported outcomes (PRO) in ovarian cancer clinical trials-lost opportunities and lessons learned. Ann Oncol. Apr. 2016;27(1):i66–i71.
Wilcox AR, Trooboff SW, Wong SL. “Evaluating Patient-Reported Outcomes in Inguinal Hernia Clinical Trials,” J. Surg. Res., vol. 244, pp. 430–435, Dec. 2019.
Schwabe MT et al. “Short-term Clinical Outcomes of Hip Arthroscopy Versus Physical Therapy in Patients With Femoroacetabular Impingement: A Systematic Review and Meta-analysis of Randomized Controlled Trials,” Orthop J Sports Med, vol. 8, no. 11, p. 2325967120968490, Nov. 2020.
Murad MH, Wang Z. “Guidelines for reporting meta-epidemiological methodology research,” Evid. Based. Med., vol. 22, no. 4, pp. 139–142, Aug. 2017.
Shepard S et al. “Post traumatic stress disorder (July 2021),” Jun. 2021, Accessed: Jul. 22, 2021. [Online]. Available: https://osf.io/mp876/
Mercieca-Bebber R et al. “Preliminary evidence on the uptake, use and benefits of the CONSORT-PRO extension,” Qual. Life Res., vol. 26, no. 6, pp. 1427–1437, Jun. 2017.
“Cochrane Training.” https://www.youtube.com/channel/UCoWzvKR8RPHG07PPeqBiibA (accessed Jul.17, 2021).
“Risk of bias tools. - Current version of RoB 2.” https://sites.google.com/site/riskofbiastool/welcome/rob-2-0-tool/current-version-of-rob-2?authuser=0 (accessed Jul.21, 2021).
“Risk of bias tools -. RoB 2 for crossover trials.” https://sites.google.com/site/riskofbiastool/welcome/rob-2-0-tool/rob-2-for-crossover-trials?authuser=0 (accessed Jul.21, 2021).
“Risk of bias tools -. RoB 2 for cluster-randomized trials.” https://sites.google.com/site/riskofbiastool/welcome/rob-2-0-tool/rob-2-for-cluster-randomized-trials?authuser=0 (accessed Jul.21, 2021).
McGauran N, Wieseler B, Kreis J, Schüler Y-B, Kölsch H, Kaiser T. “Reporting bias in medical research - a narrative review,” Trials, vol. 11, p. 37, Apr. 2010.
Weldring T, Smith SMS. “Patient-Reported Outcomes (PROs) and Patient-Reported Outcome Measures (PROMs),” Health Serv Insights, vol. 6, pp. 61–68, Aug. 2013.
“PTSD: National Center for PTSD.” https://www.ptsd.va.gov/professional/assessment/adult-sr/index.asp (accessed Jul.23, 2021).
“Chapter 5. : Collecting data.” https://training.cochrane.org/handbook/current/chapter-05 (accessed Jul. 23, 2021).
Edmond SN, Turk DC, Williams DA, Kerns RD. Considerations of trial design and conduct in behavioral interventions for the management of chronic pain in adults. Pain Rep. May 2019;4(3):e655.
Martins Scalabrin J, Mello MF, Swardfager W, Cogo-Moreira H. Risk of Bias in Randomized clinical trials on psychological therapies for post-traumatic stress disorder in adults. Chronic Stress (Thousand Oaks). Jan. 2018;2:2470547018779066.
Munder T, Barth J. Cochrane’s risk of bias tool in the context of psychotherapy outcome research. Psychother Res. May 2018;28(3):347–55.
Acknowledgements
We are grateful to April Schweikhard who assisted in the development of our search strategy and to the OSU medical library for their procurement of relevant literature. Development of this study was funded by the Oklahoma State University Center for Health Sciences Presidential Mentor-Mentee Research Fellowship Grant.
Funding
This study was not funded.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Competing Interests
No financial or other sources of support were provided during the development of this manuscript. Dr. Hartwell reports receiving funding from the National Institute of Justice for work unrelated to the current subject. Dr. Vassar reports receipt of funding from the National Institute on Drug Abuse, the National Institute on Alcohol Abuse and Alcoholism, the US Office of Research Integrity, Oklahoma Center for Advancement of Science and Technology, and internal grants from Oklahoma State University Center for Health Sciences—all outside of the present work. All other authors have nothing to report.
Additional information
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Electronic Supplementary Material
Below is the link to the electronic supplementary material.
Rights and permissions
Springer Nature or its licensor (e.g. a society or other partner) holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Hughes, G., Sutterfield, B., Anderson, R. et al. Assessment of Reporting of Patient-Reported Outcomes in Randomized Controlled Trials for Interventions of Post-Traumatic Stress Disorder. Psychiatr Q 94, 127–139 (2023). https://doi.org/10.1007/s11126-023-10017-y
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11126-023-10017-y