Abstract
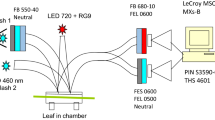
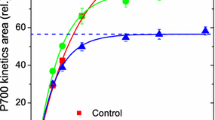
Oxygen evolution per single-turnover flash (STF) or multiple-turnover pulse (MTP) was measured with a zirconium O2 analyzer from sunflower leaves at 22°C. STF were generated by Xe arc lamp, MTP by red LED light of up to 18000 μmol quanta m−2 s−1. Ambient O2 concentration was 10–30 ppm, STF and MTP were superimposed on far-red background light in order to oxidize plastoquinone (PQ) and randomize S-states. Electron (e−) flow was calculated as 4 times O2 evolution. Q A → Q B electron transport was investigated firing double STF with a delay of 0 to 2 ms between the two. Total O2 evolution per two flashes equaled to that from a single flash when the delay was zero and doubled when the delay exceeded 2 ms. This trend was fitted with two exponentials with time constants of 0.25 and 0.95 ms, equal amplitudes. Illumination with MTP of increasing length resulted in increasing O2 evolution per pulse, which was differentiated with an aim to find the time course of O2 evolution with sub-millisecond resolution. At the highest pulse intensity of 2.9 photons ms−1 per PSII, 3 e− initially accumulated inside PSII and the catalytic rate of PQ reduction was determined from the throughput rate of the fourth and fifth e−. A light response curve for the reduction of completely oxidized PQ was a rectangular hyperbola with the initial slope of 1.2 PSII quanta per e− and V m of 0.6 e− ms−1 per PSII. When PQ was gradually reduced during longer MTP, V m decreased proportionally with the fraction of oxidized PQ. It is suggested that the linear kinetics with respect to PQ are apparent, caused by strong product inhibition due to about equal binding constants of PQ and PQH2 to the Q B site. The strong product inhibition is an appropriate mechanism for down-regulation of PSII electron transport in accordance with rate of PQH2 oxidation by cytochrome b6f.








Similar content being viewed by others
Abbreviations
- ADC:
-
Analog-to-digital converter
- ETR:
-
Electron transport rate
- F s, F m :
-
Fluorescence yields, steady state and maximum
- FRL:
-
Far-red light
- LED:
-
Light-emitting diode
- MTP:
-
Multiple-turnover pulse
- OEC:
-
Oxygen evolution complex
- PAD, PFD:
-
Photon flux density, absorbed and incident
- PGA:
-
3-Phosphoglyceric acid
- PQ(H2):
-
Plastoquinone (reduced)
- PSII:
-
Photosystem II
- RuBP:
-
Ribulose 1,5-bisphosphate
- STF:
-
Single-turnover flash
References
Berry S, Rumberg B (2000) Kinetic modeling of the photosynthetic electron transport chain. Bioelectrochemistry 53:35–53
Berry EA, Kuras MG, Huang L, Crofts AR (2000) Structure and function of cytochrome bc complexes. Annu Rev Biochem 69:1005–1075
Björkman O, Gauhl E (1970) Use of zirconium oxide ceramic cell for measurements of photosynthetic oxygen eveolution intact leaves. Photosynthetica 4:123–128
Bowes JM, Crofts AR (1980) Binary oscillations in the rate of oxidation of the primary acceptor of Photosystem II. Biochim Biophys Acta 590:373–384
Crofts AR, Wraight CA (1983) The electrochemical domain of photosynthesis. Biochim Biophys Acta 726:149–185
de Wijn R, van Gorkom HJ (2001) Kinetics of electron transfer from QA to QB in photosystem II. Biochemistry 40:11912–11922
De Wijn R, Schrama T, van Gorkom HJ (2001) Secondary stabilization reactions and proton-coupled electron transport in photosystem II investigated by electroluminescence and fluorescence spectroscopy. Biochemistry 40:5821–5834
Diner BA, Babcock GT (1996) Structure, dynamics, and energy conversion efficiency in photosystem II. In: Ort DR, Yocum CF (eds) Oxygenic photosynthesis: the light reactions. Kluwer Academic Publishers, Dordrecht/Boston/London, pp 213–247
Eichelmann H, Oja V, Rasulov B, Padu E, Bichele I, Pettai H, Mänd P, Kull O, Laisk A (2005) Adjustment of leaf photosynthesis to shade in a natural canopy: reallocation of nitrogen. Plant Cell Environ 28:389–401
Graan T, Ort DR (1984) Quantitation of the rapid electron donors to P700, the functional plastoquinone pool, and the ratio of the photosystems in spinach chloroplasts. J Biol Chem 259:14003–14010
Greenbaum E, Mauzerall DC (1976) Oxygen yield per flash of Chlorella coupled to chemical oxidants under anaerobic conditions. Photochem Photobiol 23:369–372
Haumann M, Junge W (1994) The rates of proton uptake and electron transfer at the reducing side of photodsystem II in thylakoids. FEBS Lett 347:45–50
Joliot P (1967) Oxygen exchange in algae illuminated by modulated light. Energy conversion by the photosynthetic apparatus, brookhaven symposium in biology, no. 19, pp 446–459, New York
Kirschbaum MUF, Pearcy RW (1988) Concurrent measurements of oxygen- and carbon-dioxide exchange during lightflecks in Alocasia macrorrhiza (L.) G. Don. Planta 174:527–533
Kirschbaum MUF, Oja V, A. Laisk (2005) The quantum yield of CO2 fixation is reduced for several minutes after prior exposure to darkness. Exploration of the underlying causes. Plant Biol 7:58–66
Kleinfeld D, Abresch EC, Okamura MY, Feher G (1984) Damping oscillations in the semiquinone absorption in reaction centers after successive flashes. Determination of the equilibrium between QA-QB and QAQ −B . Biochim Biophys Acta 765:406–409
Kok B, Forbush M, McGloin M (1970) Cooperation of charges in photosynthetic oxygen evolution. Photochem Photobiol 11:457–475
Kramer DM, Crofts AR (1993) The concerted reduction of the high- and low-potential chains of the cytochrome bf complex by plastoquinol. Biochim Biophys Acta 1183:72–84
Laisk A, Oja V (1976) Adaptation of the photosynthetic apparatus to light profile in the leaf. Fiziologija Rastenij (Sov Plant Physiol) 23(3):445–451 (in Russian)
Laisk A, Oja V (2000a) Alteration of PSII properties with non-photochemical excitation quenching. Phil Trans R Soc Lond B 355:1405–1418
Laisk A, Oja V (2000b) Electron transport through photosystem II in leaves during light pulses: acceptor resistance increases with nonphotochemical excitation quenching. Biochim Biophys Acta 1460:255–267
Laisk A, Oja V, Kiirats O (1984) Assimilatory power (post-illumination CO2 uptake) in leaves—measurement, environmental dependencies and kinetic properties. Plant Physiol 76:723–729
Laisk A, Kiirats O, Eichelmann H, Oja V (1987) Gas exchange studies of carboxylation kinetics in intact leaves. In: Biggins J (ed) Progress in photosynthesis research. Martinus Nijhoff Publishers, Dordrecht, the Netherlands, pp 245–252
Laisk A, Eichelmann H, Oja V (2006) C3 photosynthesis in silico. Photosynth Res 90:45–66
Laisk A, Eichelmann H, Oja V, Talts E, Scheibe R (2007) Rates and roles of cyclic and alternative electron flow in potato leaves. Plant Cell Physiol 48:1575–1588
Ley AC, Mauzerall DC (1982) Absolute absorption cross-sections for photosystem II and the minimum quantum requirement for photosynthesis in Chlorella vulgaris. Biochim Biophys Acta 680:95–106
Ley AC, Mauzerall DC (1986) The extent of energy transfer among photosystem II reaction centers in chlorella. Biochem Biophys Acta 850:234–248
Nishio JN, Sun J, Vogelmann TC (1993) Carbon fixation gradients across spinach leaves do not follow internal light gradients. Plant Cell 5:953–961
Oja V, Laisk A (2000) Oxygen yield from single turnover flashes in leaves: non-photochemical excitation quenching and the number of active PSII. Biochim Biophys Acta 1460:291–301
Oja V, Bichele I, Hüve K, Rasulov B, Laisk A (2004) Reductive titration of photosystem I and differential extinction coefficient of P700+ at 810–950 nm in leaves. Biochim Biophys Acta 1658:225–234
Paillotin G (1976) Movement of excitations in the photosynthetic domains of photosystem II. J Theor Biol 58:237–252
Pettai H, Oja V, Freiberg A, Laisk A (2005) Photosynthetic activity of far-red light in green plants. Biochim Biophys Acta 1708:311–321
Reifarth F, Christen G, Seelinger AG, Dörmann P, Benning C, Renger G (1997) Modification of the water oxidizing complex in leaves of the dgd1 mutant of Arabidopsis thaliana deficient in the galactolipid digalactosyldiacylglycerol. Biochemistry 36:11769–11776
Renger G, Hanssum B (2009) Oxygen detection in biological systems. Photosynth Res 102:487–498
Renger G, Wolff C (1976) The existence of a high photochemical turnover rate at the reaction centers of System II in Tris-washed chloroplasts. Biochim Biophys Acta 423:610–614
Stowell MHB, McPhillips TM, Rees DCSSM, Abresh E, Feher G (1997) Light-induced structural changes in photosynthetic reaction center: implications for mechanism of electron-proton transfer. Science 276:812–816
Talts E, Oja V, Rämma H, Rasulov B, Anijalg A, Laisk A (2007) Dark inactivation of ferredoxin-NADP reductase and cyclic electron flow under far-red light in sunflower leaves. Photosynth Res 94:109–120
Terashima I (1992) Anatomy of non-uniform leaf photosynthesis. Photosynth Res 31:195–212
Terashima I, Inoue Y (1984) Comparative photosynthetic properties of palisade tissue chloroplasts and spongy tissue chloroplasts of Camelia japonica L: functional adjustment of the photosynthetic apparatus to light environment within a leaf. Plant Cell Physiol 25:553–563
Vater J, Renger G, Stiehl HH, Witt HT (1968) Intermediates and kinetics in water splitting part of photosynthesis. Naturwiss 55:220–221
Vogelmann TC, Evans JR (2002) Profiles of light absorption and chlorophyll within spinach leaves from chlorophyll fluorescence. Plant Cell Environ 25:1313–1323
Walker D (1987) The use of the oxygen electrode and fluorescence probes in simple measurements of photosynthesis. Robert Hill Institute, University of Sheffield, Sheffield
Warburg O, Krippahl G (1960) Weiterentwicklung der manometrischen Methoden (Carbonatgemische). Z Naturforsch 15b:364–367
Xu Q, Baciou L, Sebban P, Gunner MR (2002) Exploring the energy landscape for Q −A to QB electron transfer in bacterial photosynthetic reaction centers: effect of substrate position and tail length on the conformational gating step. Biochemistry 41:10021–10025
Acknowledgment
This study was supported by Targeted Financing Theme SF0180045s08 from Estonian Ministry of Education and Science and Grants 8283 and 8344 from Estonian Science Foundation.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Oja, V., Eichelmann, H. & Laisk, A. Oxygen evolution from single- and multiple-turnover light pulses: temporal kinetics of electron transport through PSII in sunflower leaves. Photosynth Res 110, 99–109 (2011). https://doi.org/10.1007/s11120-011-9702-9
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11120-011-9702-9