Abstract
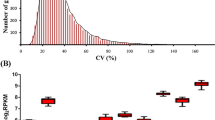
Transcriptional analysis that uncovers fruit ripening-related gene regulatory networks is increasingly important to maximize quality and minimize losses of economically important fruits such as plums. RNA sequencing (RNA-Seq) and quantitative real-time reverse transcription polymerase chain reaction (qRT-PCR) are important tools to perform high-throughput transcriptomics. The success of transcriptomics depends on the high-quality transcripts from polyphenolic- and polysaccharide-enriched plum fruits, whereas reliability of quantification data relies on accurate normalization using suitable reference gene(s). We optimized a procedure for high-quality RNA isolation from vegetative and reproductive tissues of climacteric and non-climacteric plum cultivars and conducted high-throughput transcriptomics. We identified 20 candidate reference genes from significantly non-differentially expressed transcripts of RNA-Seq data and verified their expression stability using qRT-PCR on a total of 141 plum samples which included flesh, peel, and leaf tissues of several cultivars collected from three locations over a 3-year period. Stability analyses of threshold cycle (C T) values using BestKeeper, delta (Δ) CT, NormFinder, geNorm, and RefFinder software revealed S AND protein-related trafficking protein (MON), elongation factor 1 alpha (EF1α), and initiation factor 5A (IF5A) as the best reference genes for precise transcript normalization across different tissue samples. We monitored spatiotemporal expression patterns of differentially expressed transcripts during the developmental process after accurate normalization of qRT-PCR data using combination of two best reference genes. This study also offers a guideline to select best reference genes for future gene expression studies in other plum cultivars.




Similar content being viewed by others
References
Abdi N, Holford P, McGlasson WB, Mizrahi Y (1997) Ripening behaviour and responses to propylene in four cultivars of Japanese type plums. Postharvest Biol Technol 12:21–34
Abdi N, McGlasson WB, Holford P, Williams M, Mizrahi Y (1998) Responses of climacteric and suppressed-climacteric plums to treatment with propylene and 1-methylcyclopropene. Postharvest Biology and Technology 14:29–39
Amil-Ruiz F, Garrido-Gala J, Blanco-Portales R, Folta KM, Munoz-Blanco J, Caballero JL (2013) Identification and validation of reference genes for transcript normalization in strawberry (Fragaria x ananassa) defense responses. PLoS One 8:e70603
Andersen CL, Jensen JL, Orntoft TF (2004) Normalization of real-time quantitative reverse transcription-PCR data: a model-based variance estimation approach to identify genes suited for normalization, applied to bladder and colon cancer data sets. Cancer Res 64:5245–5250
Bustin SA, Benes V, Garson JA, Hellemans J, Huggett J, Kubista M, Mueller R, Nolan T, Pfaffl MW, Shipley GL, Vandesompele J, Wittwer CT (2009) The MIQE guidelines: minimum information for publication of quantitative real-time PCR experiments. Clin Chem 55:611–622
Chang S, Puryear J, Cairney J (1993) A simple and efficient method for isolating RNA from pine trees. Plant Mol Biol Report 11:113–116
Chen D, Pan X, Xiao P, Farwell MA, Zhang B (2011a) Evaluation and identification of reliable reference genes for pharmacogenomics, toxicogenomics, and small RNA expression analysis. J Cell Physiol 226:2469–2477
Chen L, Zhong HY, Kuang JF, Li JG, Lu WJ, Chen JY (2011b) Validation of reference genes for RT-qPCR studies of gene expression in banana fruit under different experimental conditions. Planta 234:377–390
Chomczynski P, Sacchi N (1987) Single-step method of RNA isolation by acid guanidinium thiocyanate-phenol-chloroform extraction. Anal Biochem 162:156–159
Coito JL, Rocheta M, Carvalho L, Amancio S (2012) Microarray-based uncovering reference genes for quantitative real time PCR in grapevine under abiotic stress. BMC Res Notes 5:220
Czechowski T, Bari RP, Stitt M, Scheible W-R, Udvardi MK (2004) Real-time RT-PCR profiling of over 1400 Arabidopsis transcription factors: unprecedented sensitivity reveals novel root- and shoot-specific genes. Plant J 38:366–379
Czechowski T, Stitt M, Altmann T, Udvardi MK, Scheible WR (2005) Genome-wide identification and testing of superior reference genes for transcript normalization in Arabidopsis. Plant Physiol 139:5–17
de Oliveira LA, Breton MC, Bastolla FM, Camargo Sda S, Margis R, Frazzon J, Pasquali G (2012) Reference genes for the normalization of gene expression in eucalyptus species. Plant Cell Physiol 53:405–422
Die JV, Roman B (2012) RNA quality assessment: a view from plant qPCR studies. J Exp Bot 63:6069–6077
El-Sharkawy I, Kim WS, El-Kereamy A, Jayasankar S, Svircev AM, Brown DCW (2007) Isolation and characterization of four ethylene signal transduction elements in plums (Prunus salicina L.). J Exp Bot 58:3631–3643
El-Sharkawy I, Kim WS, Jayasankar S, Svircev AM, Brown DCW (2008) Differential regulation of four members of the ACC synthase gene family in plum. J Exp Bot 59:2009–2027
El-Sharkawy I, Sherif S, Mila I, Bouzayen M, Jayasankar S (2009) Molecular characterization of seven genes encoding ethylene-responsive transcriptional factors during plum fruit development and ripening. J Exp Bot 60:907–922
Exposito-Rodriguez M, Borges A, Borges-Perez A, Perez J (2008) Selection of internal control genes for quantitative real-time RT-PCR studies during tomato development process. BMC Plant Biol 8:131
Faragher JD, Brohier RL (1984) Anthocyanin accumulation in apple skin during ripening: regulation by ethylene and phenylalanine ammonia-lyase. Sci Hortic 22:89–96
Gimeno J, Eattock N, Van Deynze A, Blumwald E (2014) Selection and validation of reference genes for gene expression analysis in switchgrass (Panicum virgatum) using quantitative real-time RT-PCR. PLoS ONE 9:e91474
Giovannoni JJ (2004) Genetic regulation of fruit development and ripening. Plant Cell Online 16:S170–S180
Gonzalez-Aguero M, Garcia-Rojas M, Di Genova A, Correa J, Maass A, Orellana A, Hinrichsen P (2013) Identification of two putative reference genes from grapevine suitable for gene expression analysis in berry and related tissues derived from RNA-Seq data. BMC Genomics 14:878
Guenin S, Mauriat M, Pelloux J, Van Wuytswinkel O, Bellini C, Gutierrez L (2009) Normalization of qRT-PCR data: the necessity of adopting a systematic, experimental conditions-specific, validation of references. J Exp Bot 60:487–493
Gutierrez L, Mauriat M, Guenin S, Pelloux J, Lefebvre JF, Louvet R, Rusterucci C, Moritz T, Guerineau F, Bellini C, Van Wuytswinkel O (2008) The lack of a systematic validation of reference genes: a serious pitfall undervalued in reverse transcription-polymerase chain reaction (RT-PCR) analysis in plants. Plant Biotechnol J 6:609–618
Imai T, Ubi BE, Saito T, Moriguchi T (2014) Evaluation of reference genes for accurate normalization of gene expression for real time-quantitative PCR in Pyrus pyrifolia using different tissue samples and seasonal conditions. PLoS One 9:e86492
Kim H-Y, Farcuh M, Cohen Y, Crisosto C, Sadka A, Blumwald E (2015) Non-climacteric ripening and sorbitol homeostasis in plum fruits. Plant Sci 231:30–39
Kohl M (2007) SLqPCR: functions for analysis of real-time quantitative PCR data at SIRS-Lab GmbH. R Package, SIRS-Lab GmbH, Jena
Kozera B, Rapacz M (2013) Reference genes in real-time PCR. J Appl Genet 54:391–406
Li H, Durbin R (2009) Fast and accurate short read alignment with Burrows-Wheeler transform. Bioinforma (Oxford England) 25:1754–1760
López-Gómez R, Gómez-Lim MA (1992) A method for extracting intact RNA from fruits rich in polysaccharides using ripe mango mesocarp. HortSci 27:440–442
Mafra V, Kubo KS, Alves-Ferreira M, Ribeiro-Alves M, Stuart RM, Boava LP, Rodrigues CM, Machado MA (2012) Reference genes for accurate transcript normalization in citrus genotypes under different experimental conditions. PLoS ONE 7:e31263
McCarthy DJ, Chen Y, Smyth GK (2012) Differential expression analysis of multifactor RNA-Seq experiments with respect to biological variation. Nucleic Acids Res 40:4288–4297
Meisel L, Fonseca B, Gonzalez S, Baeza-Yates R, Cambiazo V, Campos R, Gonzalez M, Orellana A, Retamales J, Silva H (2005) A rapid and efficient method for purifying high quality total RNA from peaches (Prunus persica) for functional genomics analyses. Biol Res 38:83–88
Okie W, Ramming D (1999) Plum breeding worldwide. HortTechnology 9:162–176
Osorio S, Fernie AR (2013) Biochemistry of fruit ripening. The Molecular Biology and Biochemistry of Fruit Ripening. Blackwell Publishing Ltd., In, pp 1–19
Pech J-C, Bouzayen M, Latché A (2008) Climacteric fruit ripening: ethylene-dependent and independent regulation of ripening pathways in melon fruit. Plant Sci 175:114–120
Pfaffl MW, Tichopad A, Prgomet C, Neuvians TP (2004) Determination of stable housekeeping genes, differentially regulated target genes and sample integrity: BestKeeper—Excel-based tool using pair-wise correlations. Biotechnol Lett 26:509–515
Prasanna V, Prabha TN, Tharanathan RN (2007) Fruit ripening phenomena—an overview. Crit Rev Food Sci Nutr 47:1–19
Ramakers C, Ruijter JM, Deprez RH, Moorman AF (2003) Assumption-free analysis of quantitative real-time polymerase chain reaction (PCR) data. Neurosci Lett 339:62–66
Reid KE, Olsson N, Schlosser J, Peng F, Lund ST (2006) An optimized grapevine RNA isolation procedure and statistical determination of reference genes for real-time RT-PCR during berry development. BMC Plant Biol 6:27
Rio DC, Ares M, Hannon GJ, Nilsen TW (2010) Purification of RNA using TRIzol (TRI Reagent). Cold Spring Harbor Protocols 2010:pdb.prot5439
Ruiz-May E, Rose JKC (2013) Cell wall architecture and metabolism in ripening fruit and the complex relationship with softening. In: The molecular biology and biochemistry of fruit ripening. Blackwell Publishing Ltd., pp 163–187.
Saha P, Blumwald E (2014) Assessing reference genes for accurate transcript normalization using quantitative real-time PCR in pearl millet [Pennisetum glaucum (L.) R. Br.]. PLoS ONE 9:e106308
Saha P, Ray T, Tang Y, Dutta I, Evangelous NR, Kieliszewski MJ, Chen Y, Cannon MC (2013) Self-rescue of an EXTENSIN mutant reveals alternative gene expression programs and candidate proteins for new cell wall assembly in Arabidopsis. Plant J 75:104–116
Silver N, Best S, Jiang J, Thein SL (2006) Selection of housekeeping genes for gene expression studies in human reticulocytes using real-time PCR. BMC Mol Biol 7:33
Singh Z, Khan AS (2010) Physiology of plum fruit ripening. Stewart Postharvest Review 6:1–10
Thellin O, Zorzi W, Lakaye B, De Borman B, Coumans B, Hennen G, Grisar T, Igout A, Heinen E (1999) Housekeeping genes as internal standards: use and limits. J Biotechnol 75:291–295
Tong Z, Gao Z, Wang F, Zhou J, Zhang Z (2009) Selection of reliable reference genes for gene expression studies in peach using real-time PCR. BMC Mol Biol 10:71
Trainotti L, Zanin D, Casadoro G (2003) A cell wall-oriented genomic approach reveals a new and unexpected complexity of the softening in peaches. J Exp Bot 54:1821–1832
Trapnell C, Roberts A, Goff L, Pertea G, Kim D, Kelley DR, Pimentel H, Salzberg SL, Rinn JL, Pachter L (2012) Differential gene and transcript expression analysis of RNA-seq experiments with TopHat and Cufflinks. Nat Protocols 7:562–578
Vandesompele J, De Preter K, Pattyn F, Poppe B, Van Roy N, De Paepe A, Speleman F (2002) Accurate normalization of real-time quantitative RT-PCR data by geometric averaging of multiple internal control genes. Genome biology 3:Research0034
Wan H, Zhao Z, Qian C, Sui Y, Malik AA, Chen J (2010) Selection of appropriate reference genes for gene expression studies by quantitative real-time polymerase chain reaction in cucumber. Anal Biochem 399:257–261
Wang L, Wang Y, Zhou P (2013) Validation of reference genes for quantitative real-time PCR during Chinese wolfberry fruit development. Plant Physiol Biochemistry PPB/Soc Fri Physiol Veg 70:304–310
Wong ML, Medrano JF (2005) Real-time PCR for mRNA quantitation. BioTech 39:75–85
Zhong HY, Chen JW, Li CQ, Chen L, Wu JY, Chen JY, Lu WJ, Li JG (2011) Selection of reliable reference genes for expression studies by reverse transcription quantitative real-time PCR in litchi under different experimental conditions. Plant Cell Rep 30:641–653
Zhu X, Li X, Chen W, Chen J, Lu W, Chen L, Fu D (2012) Evaluation of new reference genes in papaya for accurate transcript normalization under different experimental conditions. PLoS ONE 7:e44405
Acknowledgments
This research was supported by the Will W. Lester Endowment of the University of California to E.B.. M.F. is a recipient of a fellowship from the Programa Formacion de Capital Humano Avanzado CONICYT, Chile. The authors are thankful to Dr. Ellen Tumimbang for technical support.
Conflict of Interest
The authors declared that they have no conflict of interest.
Author information
Authors and Affiliations
Corresponding author
Additional information
Ho-Youn Kim and Prasenjit Saha contributed equally to this work.
Electronic Supplementary Material
Below is the link to the electronic supplementary material.
Fig. S1
Illustration of plum fruit developmental changes and tissue samples used to evaluate reference genes. Fruits from developmental stages, except S1 (early stage), Stage 2 (S2, pit hardening), Stage 3 (S3, 2nd exponential growth phase) and Stage 4 (S4, ripe stage) of cultivars BB (Burbank), BG (Burgundy), DL (Dolly), EH (Elephant Heart), MT (Methley), SK (Simka), SR (Santa Rosa), SM (Sweet Miriam), QA (Queen Ann) were included in the study. See Table S1 for detailed sample description. (GIF 32 kb)
Fig. S2
Non-differential expression patterns of 20 potential reference genes from RNA-Seq analyses. Data showed log-transformed values of total RNA-Seq reads from immature stage (IS, S2) and ripe stage (RS, S4) of a climacteric cultivar (SR) and a non- climacteric cultivar (SM) in three biological replicates (R1, R2, and R3) collected during 2011. Green-yellow-red color scale depicts low-medium-high expression levels of each gene. See Table 3 for detail characteristic of candidate reference genes and Fig. S1 for developmental stages. (GIF 137 kb)
Fig. S3
Dissociation curve analyses for the conformation of specific qRT-PCR amplification from each primer pair. Melt curves showing the single peak generated after qRT-PCR using gene specific primer pair from each sample. Arrow head represents no template controls (NTC). (GIF 260 kb)
Fig. S4
Conformation of specificity of primer pairs for precise amplification of reference gene after qRT-PCR. Agarose gel showing the expected amplicon size from each primer pair after qRT-PCR of cDNAs pooled form all samples. Lane name corresponds to each reference gene used for qRT-PCR. M1 and M2 represent 50 base pair (bp) and 100 bp DNA size marker, respectively. (GIF 46 kb)
Supplementary data 1
(XLSX 1,118 kb)
Supplementary data 2
(XLSX 17 kb)
Table S1
(DOCX 17 kb)
Table S2
(DOCX 35 kb)
Table S3
(DOCX 21 kb)
Table S4
(DOCX 18 kb)
Table S5
(DOCX 28 kb)
Rights and permissions
About this article
Cite this article
Kim, HY., Saha, P., Farcuh, M. et al. RNA-Seq Analysis of Spatiotemporal Gene Expression Patterns During Fruit Development Revealed Reference Genes for Transcript Normalization in Plums. Plant Mol Biol Rep 33, 1634–1649 (2015). https://doi.org/10.1007/s11105-015-0860-3
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11105-015-0860-3