Abstract
Background
Unveiling the diversity of plant strategies to acquire and use phosphorus (P) is crucial to understand factors promoting their coexistence in hyperdiverse P-impoverished communities within fire-prone landscapes such as in cerrado (South America), fynbos (South Africa) and kwongan (Australia).
Scope
We explore the diversity of P-acquisition strategies, highlighting one that has received little attention: acquisition of P following fires that temporarily enrich soil with P. This strategy is expressed by fire ephemerals as well as fast-resprouting perennial shrubs. A plant’s leaf manganese concentration ([Mn]) provides significant clues on P-acquisition strategies. High leaf [Mn] indicates carboxylate-releasing P-acquisition strategies, but other exudates may play the same role as carboxylates in P acquisition. Intermediate leaf [Mn] suggests facilitation of P acquisition by P-mobilising neighbours, through release of carboxylates or functionally similar compounds. Very low leaf [Mn] indicates that carboxylates play no immediate role in P acquisition. Release of phosphatases also represents a P-mining strategy, mobilising organic P. Some species may express multiple strategies, depending on time since germination or since fire, or on position in the landscape. In severely P-impoverished landscapes, photosynthetic P-use efficiency converges among species. Efficient species exhibit rapid rates of photosynthesis at low leaf P concentrations. A high P-remobilisation efficiency from senescing organs is another way to use P efficiently, as is extended longevity of plant organs.
Conclusions
Many P-acquisition strategies coexist in P-impoverished landscapes, but P-use strategies tend to converge. Common strategies of which we know little are those expressed by ephemeral or perennial species that are the first to respond after a fire. We surmise that carboxylate-releasing P-mobilising strategies are far more widespread than envisaged so far, and likely expressed by species that accumulate metals, exemplified by Mn, metalloids, such as selenium, fluorine, in the form of fluoroacetate, or silicon. Some carboxylate-releasing strategies are likely important to consider when restoring sites in biodiverse regions as well as in cropping systems on P-impoverished or strongly P-sorbing soils, because some species may only be able to establish themselves next to neighbours that mobilise P.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Many global biodiversity hotspots for conservation priorities (sensu Myers et al. 2000) are located in nutrient-impoverished climatically-buffered fire-prone regions, including fynbos in South Africa (Allsopp et al. 2014; Cowling et al. 1996), kwongan in Australia (Hopper 2009; Lambers 2014) and campos rupestres, which is part of the cerrado in Brazil (Oliveira et al. 2015; Silveira et al. 2016; Villarroel Segarra and Wood 2011). Fire is a key component of all these ecosystems, and some species have evolved strategies to acquire nutrients that are released in ash during a fire (Orians and Milewski 2007). Phosphorus (P) is a key nutrient that limits primary productivity in all these environments, mainly because of their age, allowing extensive erosion and leaching over time, and lack of major rejuvenating processes (Hopper 2009; Hopper et al. 2021). The availability of soil P declines steadily with increasing soil age (Walker and Syers 1976), with further losses of P in fire-prone environments on long time scales due to convection losses and volatilisation during hot fire events (> 500˚C) (Butler et al. 2018; Giardina et al. 2000; Leitch et al. 1983; Raison et al. 1985). In addition, the low P content of parent material plays a role (Porder and Ramachandran 2013). Here, we explore some of the diversity in P-acquisition and P-use strategies that contribute to the hyperdiversity in these landscapes. We highlight the coexistence of, and interactions among, species expressing different strategies and explore how this knowledge may be applied in restoration of disturbed landscapes and in intercropping agroecosystems (Homulle et al. 2022).
We acknowledge that there are more P-acquisition strategies in P-impoverished landscapes than discussed in this review, especially carnivory, which is very common in P-impoverished habitats, coprophagy and parasitism, which is relatively uncommon in these habitats if they are seasonally dry. Since these strategies have been discussed in detail before (Cramer et al. 2014; Lambers et al. 2014; Oliveira et al. 2016), we do not deal with them in this review. Table 1 summarises the P-acquisition strategies we discuss in this review. Note that some species may express multiple strategies, depending on time since germination or since fire, or on position in the landscape.
Phosphophiles as post-fire ephemerals
When soils are relatively fertile, non-mycorrhizal plants such as most Amaranthaceae, Brassicaceae, Caryophyllaceae, Chenopodiaceae, Polygonaceae and Urticaceae are common (Brundrett 2009; Brundrett and Tedersoo 2018). Some of these non-mycorrhizal species produce abundant long root hairs, e.g., Brassica oleracea (Dechassa et al. 2003) and Spinacia oleracea (Föhse et al. 1991). For these plants, only P that is close to the surface of roots or root hairs is available (Lambers et al. 2008). These plants typically occur in nutrient-rich habitats with a high P concentration in the soil solution; they are commonly referred to as nitrophiles (Braun-Blanquet 1949), but in the present context, the term ‘phosphophiles’ is more appropriate (Lambers 2022).
In nutrient-poor, fire-prone environments, phosphophiles are common in the early stages of post-fire succession, during which a temporary (4–6 months) flush in soil P availability occurs (Brown and Mitchell 1986; Hester and Hobbs 1992). Although some P is volatilised during hot fires (> 500˚C) (Giardina et al. 2000; Raison et al. 1985), the burning of vegetation can increase plant-available P relative to pre-fire levels; burning to black carbon increases plant-available P c. 10-fold and burning to ash c. 2- to 7.5-fold (Schaller et al. 2015). Phosphophiles in these environments often represent a significant fraction of the flora (Cowling et al. 1997), with examples typically monocarpic and fast-growing fire ephemerals, such as Stipa elegantissima (Poaceae), and Macarthuria apetala (formerly Aizoaceae, but now Macarthuriaceae) in kwongan (Pate et al. 1985); and Itasina filifolia (Apiaceae) in fynbos (Rutherford et al. 2011). While many of these species complete their lifecycle within the first year post-fire, other species such as those in the genera Aspalathus (Fabaceae) (Cocks 1994), Kennedia (Fabaceae) and Comesperma (Polygalaceae) (Miller and Dixon 2014) tend to live up to five years post-fire and are referred to as multi-year fire ephemerals.
Unlike longer-lived late post-fire succession species (e.g., Cyperaceae, Proteaceae, Restionaceae), phosphophiles do not need to acquire P from poorly soluble sources that dominate in such environments (McArthur 1991; Witkowski and Mitchell 1987). Species adapted to such conditions often rely upon root specialisations such as cluster roots in Proteaceae and some Fabaceae or dauciform roots in many Cyperaceae to ‘mine’ poorly soluble P (Lambers et al. 2008). Although not commonly reported for fire ephemerals, cluster roots do occur in several legume genera, for example, Aspalathus in fynbos (MacAlister et al. 2018; Power et al. 2010), and Daviesia, Kennedia and Viminaria in kwongan (Adams et al. 2002; Lamont 1972; Nge et al. 2020); Power et al. (2010), however, found that for fynbos species, these cluster roots are relatively rudimentary. They release smaller amounts and have lower rhizosphere concentrations of carboxylates compared with most Proteaceae, resulting in a low capacity to mobilise P from sparingly soluble sources. This restriction may explain why these fire ephemerals are limited to the immediate post-fire environment where sufficient P is available to support fast growth; beyond the early stage after a fire, a capacity to fix N2 in the case of leguminous fire ephemerals offers little competitive advantage (Power et al. 2011).
The recent observation that fynbos plants belong to the world’s thinnest-rooted plant community (Lu et al. 2022) may be highly relevant for fire ephemerals in that system. These thin roots possibly function like the roots of phosphophiles such as Brassica oleracea (Dechassa et al. 2003) and Spinacia oleracea (Föhse et al. 1991), as discussed above. It is highly unlikely that these very thin roots function as scavengers long after a fire, because, as we discuss below, even mycorrhizal hyphae are ineffective at extremely low P availability. If these hyphae are ineffective, it is hard to imagine how roots that are much thicker than fungal hyphae, even the thinnest roots in the world, could be effective at acquiring P from severely P-impoverished soils (Raven et al. 2018).
Post-fire P acquisition in resprouting perennial species
In addition to the classical mycorrhizal and non-mycorrhizal P-acquisition strategies discussed below, some species appear not to strongly depend on these strategies but acquire P when it is relatively available following a fire (Giardina et al. 2000). This strategy is expressed in both non-mycorrhizal [e.g., Stirlingia latifolia (Proteaceae) (Bowen and Pate 2004), Adenanthos barbiger (Proteaceae) (Q. Shen & H. Lambers, pers. obs.)] and mycorrhizal [e.g., Macrozamia riedlei (Zamiaceae) (Grove et al. 1980), Xanthorrhoea preissii (Xanthorrhoeaceae) (Korczynskyj and Lamont 2005)] pyrophilous species. While possibly not essential, mycorrhizas might play a role in P acquisition immediately after a wildfire.
Fire is a key determinant of P cycling in P-impoverished fire-prone ecosystems, because it enhances nutrient cycling and the soil P to carbon ratio (Brown and Mitchell 1986; Giardina et al. 2000; Kutiel and Shaviv 1989). A temporary increase of the total P concentration in the top soil post-fire (Brown and Mitchell 1986) likely benefits specific species (i.e. phosphophiles) that can take up P rapidly. Brown and Mitchell (1986) showed a 26% increase of the total P concentration and the resin-extractable P concentration increased more than five-fold. These concentrations returned to pre-fire levels within four months. The strategy to quickly acquire P immediately after a fire may be exhibited in all fire-prone ecosystems but might be successful only in P-impoverished habitats, since it requires considerable plasticity in growth, as exhibited by S. latifolia (Bowen and Pate 1993). The remarkable ‘bubble roots’ exhibited by this species likely contribute to its plasticity (Fig. 1). These structures not only store starch (Bowen and Pate 1993), but also P, albeit at similar concentration as in non-bubble roots (Fig. 1). These plants only increase their biomass when nutrient (especially P) availability increases, and thus do not need a scavenging or mining strategy, because P is abundant in a short window of time (van Blerk et al. 2021). Non-mycorrhizal S. latifolia does not produce cluster roots when mature (Lambers et al. 2021), but quickly resprouts after a fire (Fig. 1a, b) and rapidly accumulates biomass, but then stops increasing in size (Bowen and Pate 1993). Leaf [Mn] in mature S. latifolia plants is low, presumably due to low carboxylate concentrations in the rhizosheath (Lambers et al. 2021). In contrast to mature plants, seedlings of S. latifolia do produce cluster roots (Fig. 2). Similarly, based on a comparison of leaf [Mn] of Stirlingia anethifolia with that of co-occurring Synaphea oligantha (Proteaceae) (Fig. 3), we surmise that this Stirlingia species functions in a similar manner to S. latifolia. The leaf [Mn] of Xanthorrhoea preissii (Zhong et al. 2021), a mycorrhizal species (Brundrett and Abbott 1991) that rapidly resprouts after a wildfire (Fig. 4), suggests it functions in a very similar manner as non-mycorrhizal Stirlingia species. The same likely pertains to mycorrhizal Kingia australis (Dasypogonaceae), Macrozamia riedlei and Xanthorrhoea gracilis which also exhibit low mature leaf [Mn] (X.M. Zhou, K. Ranathunge & H. Lambers, pers. obs.). They are among the first to resprout and grow fast after a wildfire (Fig. 5), and then their growth declines to very slow rates (Grove et al. 1980; Korczynskyj and Lamont 2005; Lamont and Downes 1979).
In the cerrado of eastern Bolivia, the newly described Plantago pyrophila (Plantaginaceae), also resprouts and flowers quickly after a fire (Villarroel Segarra and Wood 2011). The same pattern is found in Byrsonima verbascifolia (Malpighiaceae), a common shrub in the cerrado biome of Brazil that exhibits low leaf [Mn] (Lambers et al. 2015b) and resprouts as quickly as six days after fire (Fig. 6). In campos rupestres, many Velloziaceae species (Conceição et al. 2013) and lineages of Fabaceae and Melastomataceae (Simon et al. 2009) rapidly resprout and flower after a fire, and this trait is also expressed in Cyperaceae, for example, Bulbostylis paradoxa in campos rupestres (Fidelis and Zirondi 2021); Oliveira et al. (1996) found that 44 orchid species in campos rupestres flower just two weeks after fire events. Indeed, several reviews conclude that a high diversity of species exhibit post-fire flowering in cerrado (Fidelis and Zirondi 2021), kwongan and fynbos (Lamont and Downes 2011), indicating that fire is an important factor influencing flowering in these fire-prone regions. Although the links between post-fire flowering, resprouting and P-acquisition strategies are yet to be fully explored in these communities, the combination of defoliation and fertilisation, mimicking the effects of fire, significantly increases flowering (e.g., in the geophytic fynbos grass Erharta capensis; Verboom et al. 2002).
In summary, acquiring P after its availability is temporarily increased is a common strategy in fire-prone P-impoverished ecosystems. We know next to nothing about what traits are required to ensure rapid uptake of P during a short window of enhanced P availability (Box 1). We envisage that the very thin roots observed in fynbos (Lu et al. 2022) may well play a role.
Stirlingia latifolia (blueboy). (a) Habitat near Yanchep, south-western Australia, a few months after a wildfire showing vigorously resprouting plants (arrows). (b) Flowering, triggered by a fire. (c) Bubble roots. (d) Phosphorus (P) concentrations ([P]) expressed per unit dry weight (DW) in bubbles and non-bubble parts of the roots at both an unburned and a burned site. Different letters indicate significantly different means determined by ANOVA (p < 0.05). Photos: Hans Lambers (a) and Kosala Ranathunge (b, c)
Seedling of Stirlingia latifolia (blueboy) excavated from a natural habitat in Alison Baird Reserve, City of Gosnells in south-western Australia (Tauss et al. 2019), showing many cluster roots; arrowhead points towards a developing cluster and the arrow points towards a senesced cluster; scale bar = 20 mm. Photo: Kosala Ranathunge
Stirlingia anethifolia (Proteaceae). (a) Plant growing in its natural habitat in close proximity of (b) Synaphea oligantha (Proteaceae) near Hopetoun, south-western Australia. (c) Leaf manganese concentrations ([Mn]) expressed per unit dry weight (DW) of S. anethifolia growing in its natural habitat, shared with S. oligantha, used as a positive reference. Positive references comprise co-occurring species that are known to release abundant amounts of carboxylates (Zhong et al. 2021; Zhou et al. 2020). *** indicates significantly different means (p < 0.001). Photos: Hongtao Zhong
Photos of plant species that are among the first to resprout and flower after a wildfire. (a) Xanthorrhoea preissii (balga or grasstree) (Xanthorrhoeaceae); (b, c) Kingia australis (kingia) (Dasypogonaceae); photos a-c were taken in Mount Lindesay National Park, south-western Australia; (d) Macrozamia riedlei in Lesueur National Park in south-western Australia, 10 months after a fire when its entire canopy of about 40 fronds had grown back. Photos: Hans Lambers
Interestingly, Dasypogon bromeliifolius (Dasypogonaceae), another species in the same family as Kingia australis that is among the first to resprout and flower after a fire (Rudall and Conran 2012), exhibits a relatively high leaf [Mn], similar to co-occurring Banksia species, and therefore likely releases carboxylates to access P in the time between fires (Fig. 6). Cyperaceae, like Proteaceae, are typically non-mycorrhizal (Brundrett 2009; Wang and Qiu 2006) and release carboxylates, with or without producing dauciform roots (Güsewell and Schroth 2017; Masuda et al. 2021). As a result, they tend to exhibit high leaf [Mn] (Hayes et al. 2014). However, there are clear exceptions (Hayes et al. 2014), and some of them may show a similar strategy as Proteaceae that resprout vigorously after disturbance by fire, without depending on carboxylates, e.g., Lepidosperma tetraquetrum (Cyperaceae) (X.M. Zhou, K. Ranathunge & H. Lambers, pers. obs.). In campos rupestres, Bulbostylis paradoxa (Cyperaceae) flowers three days after a fire event (Fig. 5a), with inflorescences already starting to emerge within 24 h after the passage of fire (Fidelis et al. 2019). It would be worthwhile to further study Cyperaceae with low leaf [Mn] in low-P environments (Hayes et al. 2014; X.M. Zhou, K. Ranathunge & H. Lambers, pers. obs.) to determine how they acquire P.
(a) Dasypogon bromeliifolius (pineapple bush), (b) Kingia australis (kingia) (both Dasypogonaceae, growing at Karrak Reserve in Rosa Brook, south-western Australia), and (c) leaf manganese concentrations ([Mn]) expressed per unit dry weight (DW) of both Dasypogonaceae and three co-occurring Banksia species, which were used as positive references; all leaf material was collected in natural habitats of the species. Positive references comprise co-occurring species that are known to release abundant amounts of carboxylates (Zhong et al. 2021). Different letters indicate significantly different means determined by ANOVA (p < 0.05). Photos: Hans Lambers
The strategy to capitalise on nutrients released during a fire is likely confounded by other mechanisms that act together in this event. For instance, we are dealing only with resprouter species and not fire ephemerals, whose germination is triggered by karrikins in smoke (Flematti et al. 2004, 2015) or heat (Cocks and Stock 1997); we discussed these when covering phosphophile species. Resprouter species usually rely on non-structural carbohydrates to build new organs rapidly (Bowen and Pate 1993; Hansen et al. 1991; Pate et al. 1990, 1991); however, concurrent P acquisition is also essential, since P is part of the tissues. In Brazilian savannahs, fire stimulates absorptive root biomass, correlated with shoot regrowth (Oliveras et al. 2013). Variation in morphological root parameters reflect differences in soil chemistry, especially soil P and graminoid biomass changes (Le Stradic et al. 2021). To acquire P from ash, the P in the soil must be in solution. This may be the case when fire events are followed by rain, which is likely, because wildfires are usually started by lighting. Alternatively, hydraulic lift is a mechanism to bring P in solution (Pang et al. 2013) by bringing up water from deeper in the profile where plant-available P concentrations are very low (Turner et al. 2018) to shallow layers, where most of the P is located (Turner et al. 2018), especially after a fire (Giardina et al. 2000; Resende et al. 2011). Hydraulic lift is exhibited by a wide range of species (Belovitch et al. 2022). Indeed, Byrsonima verbascifolia, which resprouts very quickly after a fire exhibits hydraulic lift (Oliveira 2004). Once P is in solution and in the rhizosphere of these species, they likely take it up rapidly, but some may be sorbed onto soil particles. We have no information on the dynamics of the various fates of P in ash.
In summary, the remarkable strategy of post-fire active functional types is to switch from a typical nutrient-stress-tolerating (Grime 1977) or a K strategy (McArthur and Wilson 1967; Parry 1981) between fires, to an opportunistic ruderal (Grime 1977) or r strategy (McArthur and Wilson 1967; Parry 1981) immediately after a fire, nomenclature depending on which ecological theory is adopted. We are aware of minor shifts in strategy during plant ontogeny (Dayrell et al. 2018), but know virtually nothing about what underpins major shifts in strategy following a wildfire.
Mycorrhizas: P-scavenging strategies
The vast majority of terrestrial plant species can establish a symbiotic association with mycorrhizal fungi (Smith and Read 2008). Mycorrhizal associations can enhance plant P acquisition in moderately infertile soils (0.5-2 µM P in the soil solution), but when soils are severely P-impoverished (< 0.5 µM P in solution), mycorrhizas are less effective (Lambers et al. 2015a; Parfitt 1979) and tend to be suppressed (Abbott et al. 1984; Chu et al. 2013; Treseder and Allen 2002). The soil solutions presented by Parfitt (1979) cannot be converted into mg P kg− 1 soil, but we can compare these soil solution concentrations with those in a range of agricultural soils, representing a wide range in texture and organic matter content. The average inorganic P concentration ([Pi]) in the soil solution is 3 µM (range: 0.6 to 11 µM) (Lambers 2022). That range equates to concentrations of readily available P (i.e. resin-P, Bray-P or Colwell-P) of approximately 5 to 13 mg P kg− 1 in agricultural soils (Sandral et al. 2019; Waddell et al. 2016). Concentrations of readily available P in old kwongan soils are 0.2 to 0.6 mg P kg− 1 on low-rainfall sites (Gao et al. 2020; Laliberté et al. 2012), with rare values of about 5 mg P kg− 1 soil at high-rainfall sites (Turner et al. 2018). In fynbos, which predominantly occupies sandstone-derived soils, a similar range of 0.4 to 2.7 mg P kg− 1 occurs (Witkowski and Mitchell 1987). For a range of campos rupestres sites, soil P is about 2.6 mg P kg− 1 (Zemunik et al. 2018). That leads to the conclusion that in the range for agricultural soils (0.6 to 11 µM or 5 to 13 mg P kg− 1) mycorrhizas are expected to be effective at enhancing P acquisition. However, on the poorest sites in kwongan, fynbos and campos rupestres, mycorrhizas are likely ineffective, explaining a shift from mycorrhizal to non-mycorrhizal carboxylate-releasing strategies on the poorest sites (Zemunik et al. 2015, 2018). If mycorrhizal hyphae are ineffective at scavenging P at such a low P availability, the world’s thinnest roots found in fynbos (Lu et al. 2022), which are much thicker and shorter than fungal hyphae (Raven et al. 2018), cannot then be expected to be effective as scavengers.
An important point to make here is that the observation that a plant forms a mycorrhizal association does not provide evidence that it actually depends on mycorrhizas to acquire P (Albornoz et al. 2021). In severely P-impoverished environments, the significance of mycorrhizal associations is likely that of protection against pathogens, rather than P acquisition (Albornoz et al. 2017; Lambers et al. 2018).
Phosphorus-mining strategies based on release of low-molecular-weight exudates are associated with mobilisation of a range of other elements
Mycorrhizal symbioses are crucial to scavenge P when the soil P availability is relatively low (Smith and Read 2008). However, as discussed above, mycorrhizas are rather ineffective when soil P availability is very low (Lambers et al. 2015a; Parfitt 1979). Under such conditions, P-mining strategies are more effective; an aspect of these P-mining strategies involves the release of P-mobilising low-molecular weight carboxylates or molecules with similar effects (Lambers et al. 2018; Nagarajah et al. 1970). Here we discuss how P-mobilising exudates not only release P, but also a range of other elements. For Mn and silicon (Si), this has been explored before (de Tombeur et al. 2021a; Lambers et al. 2015b), but here we present evidence that it is likely equally relevant for a range of other elements.
Phosphorus-mining strategies include cluster roots in Proteaceae (Delgado et al. 2014; Purnell 1960; Shane and Lambers 2005), Fabaceae (Allsop and Stock 1993; Brundrett and Abbott 1991; Gardner et al. 1981; Lamont 1972) and a range of actinorhizal families (Hurd and Schwintzer 1996; Louis et al. 1990; Reddell et al. 1997), dauciform roots in many Cyperaceae (Güsewell 2017; Selivanov and Utemova 1969; Shane et al. 2006), capillaroid roots in Restionaceae and Anarthriaceae (Hayes et al. 2014; Lambers et al. 2019; Lamont 1982), sand-binding roots in Haemodoraceae and a range of other families (Abrahão et al. 2014; Hayes et al. 2014; Smith et al. 2011), vellozioid roots in Velloziaceae (Abrahão et al. 2020; Teodoro et al. 2019), and carboxylate-releasing roots without obvious specialised structures, e.g., in Cicer arietinum (Fabaceae) (Neumann and Römheld 1999; Pang et al. 2018), Vicia faba (Fabaceae) (Li et al. 2013; Wen et al. 2019), Lotus corniculatus (Fabaceae) (Kidd et al. 2018) and Kennedia (Fabaceae) (Pang et al. 2010; Ryan et al. 2012), Artemisia (Asteraceae) and Potentilla (Rosaceae) species (Tian et al. 2021). The discovery of cluster roots in species or genera previously unknown to produce these specialised structures continues, e.g., in the Daviesia group (Mirbelioids; Fabaceae) (Lambers et al. 2019; Nge et al. 2020). Likewise, functionally-equivalent structures continue to be discovered, most recently in Cactaceae (Abrahão et al. 2014) and Velloziaceae (Abrahão et al. 2020; Teodoro et al. 2019).
The recent observation that fynbos plants belong to the world’s thinnest-rooted plant community (Lu et al. 2022) may be relevant in the context of a P-mining strategy as well. We have no information on ultrathin roots in campos rupestres, but we do know that Discocactus placentiformis (Cactaceae) is a non-mycorrhizal species that produces abundant root hairs from roots with 200 μm diameter that are more than 1 mm long (Abrahão et al. 2014). These root hairs are thinner than ultrathin fynbos roots. Discocactus placentiformis exhibits a very high shoot [Mn] and plants grown in nutrient solution release carboxylates; hence it exhibits a mining, rather than a scavenging strategy. Likewise, Persoonia (Proteaceae), a large genus of 101 species, lacks cluster roots, but Persoonia species in kwongan produce abundant long root hairs (P.E. Hayes, unpubl.) and show high leaf [Mn] (Lambers et al. 2021). Again, these ultrathin structures convey a mining strategy, rather than being important to a scavenging approach. Capillaroid roots in Restionaceae are also ultrathin and express a mining strategy (Lambers et al. 2019).
In contrast to numerous angiosperms that release carboxylates either from specialised root structures or from non-specialised roots, as discussed above, Poaceae generally do not release large amounts of carboxylates (Lambers et al. 2018). There are exceptions among Poaceae, e.g., Avena sativa (Wang et al. 2016, 2018) and Sorghum bicolor (Leiser et al. 2014; Magalhaes et al. 2007). Release of carboxylates in small quantities tends to be associated with aluminium (Al) resistance, e.g., malate release in Triticum aestivum (Poaceae) (Delhaize et al. 1993) and citrate and malate in a Eucalyptus (Myrtaceae) clone (Li et al. 2021). However, Poaceae, which exhibit Strategy II to acquire iron (Fe) (Lambers and Oliveira 2019), do release phytosiderophores, generally in response to a low availability of Fe or zinc (Zn) (Ma 2005; Römheld and Schaaf 2004), but they also mobilise Mn (Zhang 1993). Microlaena stipoides (Poaceae) is a perennial grass with high leaf [Mn] when grown in a low-P habitat (X.M. Zhou, K. Ranathunge & H. Lambers, pers. obs.), but it does not release carboxylates in a low-P nutrient solution. Rather, this grass releases phytosiderophores when Fe sufficient, but P starved (X.M. Zhou, K. Ranathunge & H. Lambers, pers. obs.), suggesting that phytosiderophores may play a role in P acquisition under low-P conditions. Since Fe-deficient Zea mays (Poaceae) plants accumulate more P in their roots and shoots than Fe-sufficient ones (Zanin et al. 2017), phytosiderophores likely enhance P availability in soil. Because much inorganic P in soil is sorbed onto oxides and hydroxides of Fe in acid soils (Barrow et al. 2021), we may expect phytosiderophores to mobilise both Fe and P, as well as Mn, but this requires further investigation (Box 1).
Di- and tricarboxylates are effective at desorbing P sorbed onto oxides and hydroxides of Al and Fe, mobilising Al and Fe at the same time (Earl et al. 1979; Geelhoed et al. 1998; Lopez-Hernandez et al. 1979). It is therefore not surprising that there is a strong correlation between leaf [P] and leaf [Al] in carboxylate-releasing Banksia species (Proteaceae) grown in a range of soils collected in natural Banksia habitats (Fig. 7). The slope of the regression lines strongly depends on the species, because some Banksia species, e.g., B. laricina, are clearly Al-accumulating species, whereas others, e.g., B. chaemaephyton, exclude Al. For Fe, no such correlation is found, because Fe uptake in plants tends to be tightly regulated (Baxter et al. 2008; Jeong and Guerinot 2009). This tight regulation thus avoids Fe toxicity (Fageria et al. 2008). Although Fe acquisition is also tightly controlled in Banksia species, unlike that in non-graminaceous plants (i.e. typical Strategy I species (Ma 2005)), this control in Banksia is not based on regulation of Fe reductase (Cawthray et al. 2021). This finding challenges the model that is commonly accepted for species that exhibit Strategy I to acquire Fe.
In cerrado, Miconia albicans (Melastomataceae) accumulates large amounts of Al in its fruits (Pasta et al. 2019). This plant likely exhibits a carboxylate-releasing P-mobilising strategy, as do other Al-accumulating species in cerrado (Amaury de Medeiros and Haridasan 1985; de Andrade et al. 2011; Haridasan and De Araújo 1988).
A carboxylate-releasing strategy may well play a role in other severely P-impoverished systems in the tropics, but we have very little hard evidence to draw a strong conclusion (Box 1). The recent observation that many species (51 species in 24 genera, belonging to 12 families) in tropical forests on P-impoverished soils in Borneo hyperaccumulate Mn (van der Ent et al. 2019) suggests that these likely depend on carboxylate release to acquire P. One might also expect carboxylate-releasing strategies to be pervasive in Amazonia, but this has yet to be explored (Reichert et al. 2022).
We envisage that many species that accumulate Si, which is co-mobilised by P-mobilising carboxylates (de Tombeur et al. 2021a), will likely release carboxylates, e.g., Equisetum species (Equisetaceae) (Hodson and Evans 1995; Husby 2013) and Phyllostachys heterocycla (Poaceae) (Lux et al. 2003); alternatively, phytosiderophores may mobilise Si in Poaceae (Gattullo et al. 2016). The acquisition of P from the C horizon by Equisetum in an Alaskan shrub wetland brings it to the soil surface, increasing the amount of P in the O horizon (Marsh et al. 2000). This ability of Equisetum to act as a nutrient pump, and its accumulation of Si might be accounted for by carboxylate release.
Carboxylates may also be indirectly involved in mobilising non-essential elements. For example, species that accumulate fluoroacetate may be expected to release P-mobilising carboxylates, because the fluoride (F) availability in the natural habitats of these species tends to be very low (Twigg and King 1991; Vickery and Vickery 1972). Carboxylates may mobilise F from substrates that have low F availability. Examples of genera that comprise such species include Gastrolobium (Fabaceae) in south-western Australia (Aplin 1969; Twigg 2014), Amorimia (Malpighiaceae), Arrabidaea (Bignoniaceae) and Palicourea (Rubiaceae) in Brazil (Cook et al. 2014; Krebs et al. 1994; Lee et al. 2012) and Dichapetalum (Dichapetalaceae) in southern Africa (Peters et al. 1960; Vickery and Vickery 1972). Palicourea rigida (Rubiaceae) is one of the species in its genus that does not accumulate fluoroacetate (Cook et al. 2014). Its leaf [Mn] is low compared with that of other species in the same habitat (< 50 mg kg− 1) (Lambers et al. 2015b), but it is the only Palicourea species for which leaf [Mn] is available. de Tombeur et al. (2021b) reported low leaf [Mn] for both Gastrolobium linearifolium and G. nervosum, but for neither is toxicity due to fluoroacetate known (Chandler et al. 2002). The leaf [Mn] in the highly toxic G. bilobum (Chandler et al. 2002) is high (100 mg kg− 1), compared with the negative reference but not as high (200 mg kg− 1) as that of the cluster-rooted positive reference (Fig. 8). This indicates that G. bilobum depends on a carboxylate-releasing P-mobilising strategy. Further studies on leaf [Mn] and fluoroacetate accumulation in other Gastrolobium species as well as species in other fluoroacetate-bearing genera are needed to test the hypothesis that carboxylates play a role in F mobilisation.
Gastrolobium bilobum (heart-leaf poison; Fabaceae), which grows on soils with a low fluoride (F) availability and accumulates F as the highly toxic fluoroacetate (Chandler et al. 2002; Twigg 2014). (a) A tree in its natural habitat in Roley Pool Reserve near Perth in south-western Australia; (b) flowers and (c) developing fruits of G. bilobum. (d) Leaf manganese concentrations ([Mn]) expressed per unit dry weight (DW) of G. bilobum and two references species, Hakea prostrata (Proteaceae) as positive and Xanthorrhoea preissii (Xanthorrhoeaceae) as negative reference. The positive reference is known to release abundant amounts of carboxylates (Shane et al. 2004a), whereas the negative reference is a species that releases very few carboxylates (Zhong et al. 2021). Different letters indicate significantly different means determined by ANOVA (p < 0.05). Photos: Hans Lambers
DeGroote et al. (2018) suggested that hyperaccumulation of Mn in Phytolacca americana (Phytolaccaceae) may be a side effect of a P-acquisition mechanism, rather than an adaptation in its own right. Likewise, Astragalus (Fabaceae) and Neptunia (Fabaceae) species that hyperaccumulate selenium (Se) (Pinto Irish et al. 2021; Sors et al. 2005), and Anacardium occidentale (Anacardiaceae) that is known for its high [Se] in both reproductive (edible) and vegetative parts (Lim 2012), might take up Se because they mobilise it as a result of the release of carboxylates under P deficiency (Lan et al. 2012). Although Se is not an essential plant nutrient, it is sometimes considered a beneficial element (Silva et al. 2020). It may act as an antioxidant in plants at low concentrations (1 to 10 µg Se g− 1 dry weight) because of its capacity to enhance the activity of radical-scavenging enzymes and the synthesis of non-enzymatic antioxidant compounds (Chauhan et al. 2019). The possible role of carboxylates in mobilising Se also warrants further investigation.
We surmise that species that hyperaccumulate elements such as Al, nickel (Ni), Zn, cadmium (Cd), Mn, arsenic (As), Se or rare earth elements (Li et al. 2018; Liu et al. 2021; Severne and Brooks 1972; Van der Ent et al. 2013, 2019; Webb 1954) do so because these elements are co-mobilised by carboxylates or functionally similar compounds that are released as a strategy to mobilise and acquire P. This is in line with results reviewed by de Tombeur et al. (2021a) on co-mobilisation of Si and P. This is not to say that all species that release carboxylates and co-mobilise metals and Si will accumulate these elements, because their uptake may involve transporters that are either not very specific (Baxter et al. 2008) or require specific transporters that do not occur in all species, as for Si (Coskun et al. 2019). When these transporters are not specific, accumulation is expected; when specific transporters are required, we only expect accumulation in species that express these specific transporters.
Phosphorus-mining strategies based on the release of phosphatases
Some plants access organic P, following release of acid phosphatases, without concomitant release of carboxylates. This is particularly prominent in Fabaceae (Houlton et al. 2008; Olde Venterink 2011), including Fabaceae in severely P-impoverished habitats (Png et al. 2017). This strategy is only effective when the organic P in soil is relatively mobile, e.g., breakdown products of phospholipids and nucleic acids (Doolette et al. 2011; Zhong et al. 2021), and would not work to access phytate, which strongly sorbs to soil particles (Anderson et al. 1974; Turner 2007). For example, in P-impoverished soil, Adenanthos cygnorum (Proteaceae), which only produces short-lived tiny ineffective cluster roots, can hydrolyse organic P that is less tightly bound by releasing acid phosphatases without large amounts of carboxylates (Q. Shen & H. Lambers, pers. obs.). Interestingly, this species exhibits an alternative P-acquisition strategy in more severely P-impoverished soil. Rather than producing cluster roots, it is facilitated by neighbouring cluster-root producing Banksia attenuata to acquire P (Q. Shen & H. Lambers, pers. obs.).
Facilitation by P-mobilising neighbours
Facilitation occurs when one plant enhances the growth, survival and/or fitness of another plant (Callaway 1995; Fletcher et al. 2016). Facilitation based on P mobilisation by carboxylates released by P-efficient neighbours is increasingly acknowledged as a strategy to acquire P by P-inefficient neighbours (Lambers et al. 2018; Li et al. 2014; Yu et al. 2021). A promising way to explore this kind of facilitation is by comparing leaf [Mn] of targeted plants with those of neighbouring reference species (Muler et al. 2014; Zhou et al. 2020). Reference species should include both a positive reference, known to release large amounts of carboxylates and to have high leaf [Mn], and a negative reference that releases virtually no carboxylates and has low leaf [Mn] (Zhong et al. 2021; Zhou et al. 2020). Based on this approach, it is likely that P uptake in Agonis flexuosa (Myrtaceae) in south-western Australia which does not release carboxylates when P availability is low (Huang et al. 2017), is facilitated, possibly by Anarthriaceae, Cyperaceae and Restionaceae in the understorey (Fig. 9). Likewise, Bossiaea species (Fabaceae) that do not release carboxylates are likely facilitated in a similar manner, possibly by Proteaceae (Abrahão et al. 2018). Interestingly, Bossiaea aquifolium exhibits a high leaf [Mn], when compared with a positive reference (Banksia grandis) and a negative reference (Xanthorrhoea preissii) (X.M. Zhou, K. Ranathunge & H. Lambers, pers. obs.), so it likely releases P-mobilising carboxylates. Hibbertia hypericoides (Dilleniaceae) is also likely facilitated by Proteaceae (Zhong et al. 2021). When digging around its roots, it is common to find cluster roots of an adjacent Proteaceae (Fig. 10). In overgrazed Inner Mongolian steppes, Cyperaceae function as facilitators of some grasses (Yu et al. 2020a, b). Likewise, when these systems are fertilised with N, mimicking N deposition in acid rain, carboxylate release from Artemisia frigida (Asteraceae) will likely facilitate P uptake in some neighbouring grasses (Tian et al. 2021). Since not all grasses are facilitated in these systems, the facilitated species are also exhibiting a strategy; their traits must somehow match those of the facilitator. When roots of Cleistogenes squarrosa and Bromus inermis (both Poaceae) interact with a facilitating neighbour, they tend to show greater plasticity of root proliferation or rhizosheath acid phosphatase activity compared with other non-P-mobilising species (Yu et al. 2020a). Greater variation in these root traits strongly correlates with increased performance in the presence of a facilitator. In rhizobox experiments involving two species, Hibbertia racemosa (Dilleniaceae) exhibits more root growth towards its carboxylate-releasing Banksia attenuata (Proteaceae) neighbour than towards another Hibbertia racemosa plant (de Britto Costa et al. 2021). The facilitated plants may be responding to highly mobile allelochemicals released from the facilitator (Delory et al. 2016; Kong et al. 2018; Li et al. 2020) or microorganisms in its rhizosphere (Peñuelas et al. 2014; van Dam and Bouwmeester 2016), but we do not know what these signals might be. Thus, we can consider plasticity in specific root traits or directing growth towards a facilitator as a strategy to acquire P mobilised by a P-efficient neighbour, rather than concluding that these species lack a strategy (Yu et al. 2021).
A severely phosphorus (P)-impoverished habitat on a c. 2-million-year-old dune along the Warren chronosequence near Pemberton in south-western Australia (Turner et al. 2018). (a) Agonis flexuosa (Western Australian peppermint, yellow horizontal arrow) (Myrtaceae) is a significant overstorey tree that is likely facilitated by species in the understorey, for example Anarthriaceae, Cyperaceae or Restionaceae (white vertical arrows) (Huang et al. 2017). (b), (c), (d) Details of A. flexuosa. (e), (f) Female flowers of Anarthria scabra (Anarthriaceae), a significant component of the understorey. Photos: a, c-f: Hans Lambers; b; Graham Zemunik
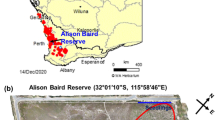
Hibbertia hypericoides (Dilleniaceae) surrounded by cluster roots (pointed at by arrows) produced by a Banksia species (Proteaceae) in its natural habitat in Alison Baird Reserve in the City of Gosnells, south-western Australia (Tauss et al. 2019). Photo: Kosala Ranathunge
Facilitation of P acquisition by carboxylate-releasing species may be indirect, as proposed for southern South American species on rich volcanic soils with low P availability (Lambers et al. 2012a). This facilitation involves shedding P-rich litter, and neighbours accessing organic P mediated by exuded or microbial phosphatases. This strategy can be expected to work only if the organic P is not strongly sorbed onto litter or soil. To access compounds like phytate, which strongly sorbs onto soil (Anderson et al. 1974; Turner et al. 2002), release of phosphatases or phytases alone would not work (Giles et al. 2017). However, in highly-weathered soils, very little organic P occurs as phytate (Turner et al. 2014; Zhong et al. 2021), and the organic P compounds that occur in these soils are more readily hydrolysed by rhizosheath phosphatases (Zhong et al. 2021).
Phosphorus-use efficiency: photosynthetic P-use efficiency
Whereas plants may express a range of divergent strategies to acquire P in habitats with low P availability, they appear to converge when it comes to their photosynthetic P-use efficiency (Denton et al. 2007a; Guilherme Pereira et al. 2019; Lambers et al. 2012b; Sulpice et al. 2014) and P-remobilisation efficiency and proficiency (Denton et al. 2007a; Guilherme Pereira et al. 2019; Hayes et al. 2014; Tsujii et al. 2017b; Zhong et al. 2021). Photosynthetic P-use efficiency (PPUE) is the rate of carbon-fixation per unit leaf P; a higher PPUE indicates a more efficient use of P for photosynthesis. Species from severely P-impoverished habitats tend to have a very low total leaf [P] (~ 0.3 mg g− 1 in south-western Australia), yet maintain photosynthetic rates similar to many crop plants, thus achieving a high PPUE (Guilherme Pereira et al. 2019). Along the Jurien Bay chronosequence in south-western Australia, PPUE is much higher in species on the oldest most severely P-impoverished sites (~ 200 µmol CO2 g− 1 P s− 1), compared with that in species on younger P-richer sites (~ 90 µmol CO2 g− 1 P s− 1) (Guilherme Pereira et al. 2019). This is consistent across a range of species, indicating convergence in P-use efficiency. In contrast with the variation in P-acquisition strategies, convergent P-use strategies have been identified across a range of P-efficient species, including low mature leaf lipid-P concentrations, achieved through lipid remodelling, low investment in ribosomal RNA (rRNA), low inorganic-P concentrations, and a preferential allocation of P to photosynthetically active mesophyll cells (Guilherme Pereira et al. 2018; Hayes et al. 2018; Lambers et al. 2012b; Sulpice et al. 2014).
Lipid remodelling is the substitution of phospholipids with lipids that do not contain P (e.g., sulfolipids or galactolipids), thus reducing total leaf P (Tjellström et al. 2008). Phosphorus-efficient Proteaceae from south-western Australia show high levels of lipid remodelling during leaf development, thus reducing their mature leaf [P] without compromising photosynthetic capacity (Kuppusamy et al. 2014; Lambers et al. 2012b). In rice (Oryza sativa (Poaceae)) plants grown under low-P conditions, a low investment in phospholipids is strongly associated with a high PPUE, again, with no reduction in photosynthetic capacity (Hayes et al. 2022). We surmise that lipid remodelling has little impact on photosynthesis, because most phospholipids are found outside the chloroplasts and can be remodelled without impacting photosynthesis (Mamode Cassim et al. 2019; Nakamura 2017).
Nucleic acid P (predominantly rRNA) is generally the largest leaf fraction of organic P in plants grown at adequate P supply; 30% in Hordeum vulgare (barley; Poaceae) (Chapin and Bieleski 1982), 40% in Oryza sativa (Jeong et al. 2017) and as high as 50% in rice grown under low-P conditions (Hayes et al. 2022). The latter increase in relative allocation under low-P conditions reflects a reduction in other P fractions (mainly lipid P and inorganic P), rather than an increase in nucleic acid P (Hayes et al. 2022; Jeong et al. 2017). Phosphorus-efficient species allocate ~ 30–40% of total P to nucleic-acids, but this represents a very low concentration, < 0.1 mg g− 1 (Melaleuca systena (Myrtaceae) and Hakea prostrata); thus investment in nucleic acid P (predominantly rRNA) is very low in highly P-efficient species (Sulpice et al. 2014; Yan et al. 2019). A low investment in rRNA significantly reduces total leaf [P], but is not associated with slow rates of photosynthesis; however, it may result in slower rates of protein turnover, which may impact the plant’s ability to rapidly respond to environmental stresses (Lambers 2022). This needs further investigation.
Inorganic phosphate is often the largest P fraction when P supply is abundant. It closely reflects P supply in a range of crop and pasture plants such as Brassica napus (Brassicaceae), Cucurbita maxima (Cucurbitaceae) (Pant et al. 2008), Oryza sativa (Hayes et al. 2022; Jeong et al. 2017), Medicago truncatula (Fabaceae) (Branscheid et al. 2010), as well as plants such as Hakea prostrata and Melaleuca systena (Shane et al. 2004b; Yan et al. 2019) that are native to south-western Australia. Rice plants grown under low-P conditions show a significant decrease in inorganic-P concentration, from 30 to 18% of total P (Hayes et al. 2022), and similar reductions occur in field-collected Melaleuca systena and Hakea prostrata, when compared across sites of decreasing soil P availability within their natural range (Yan et al. 2019).
In addition to efficient investment of P into chemical P fractions, it is also important to consider where within a leaf P is actually invested. Phosphorus is preferentially allocated to specific leaf cells (Conn and Gilliham 2010). Many dicots allocate leaf P preferentially to epidermal cells (Conn and Gilliham 2010), whereas eudicots from severely P-impoverished habitats (Guilherme Pereira et al. 2018; Hawkins et al. 2008; Hayes et al. 2018; Shane et al. 2004b) and monocots (Boursier and Läuchli 1989; Dietz et al. 1992; Karley et al. 2000) preferentially allocate P to mesophyll cells. By preferentially allocating P to where it is needed in the greatest amount, photosynthetically active mesophyll cells, rather than metabolically inactive epidermal cells, these species are more P-efficient (Stitt et al. 2010; Tsujii et al. 2017a). This efficient P allocation is not restricted to species from kwongan, cerrado and fynbos (Guilherme Pereira et al. 2018; Hayes et al. 2018; Lambers et al. 2015a), but has also been found in a tropical tree species from a P-impoverished site in Borneo (Tsujii et al. 2017a). Proteaceae from severely P-impoverished south-western Australia (high PPUE) allocate P to mesophyll cells, whereas Proteaceae from P-richer regions in Chile (low PPUE) do not (Hayes et al. 2018), with similar observations made for other eudicot families (Guilherme Pereira et al. 2018). This P-use strategy therefore reflects the habitat in which species have evolved, rather than their phylogeny, and is an excellent example of a common P-use strategy in P-efficient species.
Phosphorus-use efficiency: P-remobilisation efficiency
A high remobilisation of P from senescing organs (mainly leaves) is important to reduce P loss, making that P available for growth elsewhere in the plant and preventing its loss to the environment, increasing overall P-use efficiency. Leaf P-resorption efficiency is the proportion of P resorbed, while P-resorption proficiency is the final concentration to which P is reduced in senescing leaves (Aerts and Chapin 1999; Killingbeck 1996). High levels of P-resorption efficiency and proficiency are common among species from severely P-impoverished systems, with as much as 90% being resorbed from senescing leaves in highly P-efficient Proteaceae and a community average of 79% in the most P-limited stage of the Jurien Bay chronosequence (south-western Australia) (Denton et al. 2007a; Hayes et al. 2014). Furthermore, species that preferentially allocate P to mesophyll cells also tend to have a more efficient P remobilisation, suggesting a possible link between these two strategies and emphasising the commonality of these traits among species from P-impoverished systems.
Results on cerrado species indicate a highly efficient P remobilisation, supporting the idea that cerrado plants, like those in fynbos and kwongan, are limited by P (Kozovits et al. 2007). However, that does not mean that P addition can be expected to enhance the productivity of individual plants in this system, as Lu et al. (2022) hypothesised for fynbos plants. Rather, P addition shifts the ecosystem, replacing slow-growing highly P-efficient species by faster-growing less efficient ones (Specht 1963). Heath species are excluded from more fertile soils by harmful effects on seedlings (P toxicity; Lambers et al. 2013; Nichols et al. 1979) and competition from more vigorous herbaceous plants in this environment (Heddle and Specht 1975).
In tropical tree species on Mount Kinabalu, Borneo, at severely P-impoverished sites, P-remobilisation efficiency was 93% in species from the most severely P-impoverished site (Tsujii et al. 2017b). Phosphorus remobilisation was greatest from the phospholipid and nucleic acid fractions, and least for the easily soluble fraction, possibly because breakdown of phospholipids and nucleic acids produces compounds captured in the easily soluble fraction. The residual fraction was also remobilised to a smaller extent. For species with higher P-remobilisation efficiency, resorption from the residual fraction was relatively high and similar in magnitude to that of labile fractions. This suggests that tree species inhabiting P-impoverished habitats increase their P-remobilisation efficiency by greater degradation of recalcitrant compounds, which are likely phosphorylated proteins (Tsujii et al. 2017b).
Phosphorus-use efficiency: leaf longevity
Whilst PPUE gives an indication of instantaneous P-use efficiency and P remobilisation provides insight into how much P is remobilised from senescing organs to be used elsewhere in the plant, as opposed to being lost in litter, neither provides information on the use of P over the lifetime of a leaf. To capture this, we need to know how long a leaf functions and uses the P invested in it. A low PPUE and P-remobilisation efficiency can be compensated by a long leaf longevity (Berendse and Aerts 1987). Leaf longevity varies substantially among species growing at the same site in kwongan. For a Banksia woodland with a kwongan understorey Veneklaas and Poot (2003) found an average leaf longevity of 2.8 years for trees (deep-rooted), 3.1 years for other deep-rooted species, and 1.7 years for shallow-rooted species. The highest value was found for Macrozamia riedlei (Zamiaceae), 8.8 years, compensating for its low PPUE (57 µmol CO2 g− 1 P s− 1) because of its modest rates of photosynthesis (11.6 µmol CO2 m− 2 s− 1) and relatively high leaf [P] (600 µg g− 1 DW) (P.E. Hayes and H. Lambers, unpubl.).
Perspectives and knowledge gaps
The diversity of P-acquisition and P-use strategies is one aspect contributing to the hyperdiversity in biodiversity hotspots. Many of these hyperdiverse systems are fire-prone and some species express strategies to acquire P after a fire that temporarily increases the soil P availability (Fig. 11). While fire is able to provide a temporary flush in soil P, we know very little about the dynamics of soil P in the first few months after a fire and what root traits allow rapid access to P in ash (Box 1; Fig. 12).
Phosphorus (P)-acquisition strategies as dependent on soil age and time since fire in ancient landscapes. The changes in soil P with soil age are based on Walker and Syers (1976) as modified by Turner and Condron (2013). The P-acquisition strategies and effects of fire on soil P are discussed in detail in this review. Note that some species may express multiple strategies, for example, dependent on their developmental stage, time since a major disturbance such as fire, or location in the landscape, as discussed in this review

Variation in plant-available phosphorus concentrations ([P]), measured as resin-P, expressed on a soil dry weight (DW) basis with time after fire, and soil depth in the < 2-mm fraction at a sandplain lowland fynbos location (Pella, South Africa). Reproduced with permission, with minor modification, from Brown and Mitchell (1986)
The P-acquisition strategies discussed in this review are equally relevant for ecosystems where P is increasingly becoming limiting for primary productivity, because of either overgrazing (Yu et al. 2020a, b), atmospheric N deposition (Tian et al. 2021, 2022) or global warming (Zhou et al. 2021). Many highly P-efficient species are excluded from more fertile soils by harmful effects on seedling growth (Lambers et al. 2013; Nichols et al. 1979) and competition from more vigorous herbaceous plants in this environment (Heddle and Specht 1975). A recent global meta-analysis revealed that P limitation of aboveground primary productivity in natural terrestrial ecosystems is far more common than widely acknowledged (Hou et al. 2020). Therefore, P-acquisition mechanisms, including those that maximise benefits resulting from facilitation based on P-mobilisation by neighbours, are likely pervasive and worth further consideration. Facilitation of a plant’s P uptake by P-mobilising neighbours is a P-acquisition strategy in itself, and we still understand very little about the root traits that favour facilitation and what allows a plant to preferentially position its roots near those of a facilitating neighbour (de Britto Costa et al. 2021) (Box 1).
Analyses of leaf [Mn] as a proxy for rhizosphere carboxylate concentrations or functionally similar compounds in combination with glasshouse studies on selected species to verify the proxy, provide a novel tool to explore belowground interactions (Lambers et al. 2021; Zhong et al. 2021; Zhou et al. 2020). This has led to the conclusion that phytosiderophores, which are well known to be released by grass roots and to mobilise Fe, Zn and Mn, are also released in response to P deficiency in Microlaena stipoides (Poaceae) (X.M. Zhou, K. Ranathunge & H. Lambers, pers. obs.). We do not know how widespread this strategy is among grasses and how it is controlled by P deficiency (Box 1).
The interactions we discussed when focusing on P-impoverished landscapes are also highly relevant in agroecosystems based on intercropping (Dowling et al. 2021; Homulle et al. 2022; Li et al. 2014). As we are beginning to understand the subtleties of belowground interactions involving P-acquisition strategies (Yu et al. 2021), we can work towards optimisation of combinations of crop species and genotypes (Cong et al. 2020; Dowling et al. 2021). When aiming to restore disturbed sites in landscapes where P availability is very low, for example after mining or farming, the mechanisms discussed in this review, especially those focusing on facilitation of P acquisition based on carboxylate release, are highly relevant. Without suitable facilitators, species that depend on facilitation may never make it and hence be lacking from restored sites. It will be a challenge to identify suitable combinations of facilitators and facilitates species (Box 1).
In summary, in ancient landscapes there are a range of P-acquisition strategies that plants exhibit, some of which have been given little attention so far, especially those involving the use of P from ash (Box 1) and P released by facilitators (Box 1). Understanding this variation of strategies will inform management and restoration of hyperdiverse systems in P-impoverished fire-prone landscapes and contribute to greater P-use efficiency in managed landscapes.
References
Abbott LK, Robson AD, De Boer G (1984) The effect of phosphorus on the formation of hyphae in soil by the vesicular-arbuscular mycorrhizal fungus Glomus fasciculatum New Phytol 97:437–446. https://doi.org/10.1111/j.1469-8137.1984.tb03609.x
Abrahão A, de Britto Costa P, Teodoro GS, Lambers H, Nascimento DL, Andrade SAL, Ryan MH, Oliveira RS (2020) Vellozioid roots allow for habitat specialization among rock- and soil-dwelling Velloziaceae in campos rupestres. Funct Ecol 34:442–457. https://doi.org/10.1111/1365-2435.13479
Abrahão A, Lambers H, Sawaya A, C H F, Mazzafera P, Oliveira RS (2014) Convergence of a specialized root trait in plants from nutrient-impoverished soils: phosphorus-acquisition strategy in a nonmycorrhizal cactus. Oecologia 176:345–355. https://doi.org/10.1007/s00442-014-3033
Abrahão A, Ryan MH, Laliberté E, Oliveira RS, Lambers H (2018) Phosphorus- and nitrogen-acquisition strategies in two Bossiaea species (Fabaceae) along retrogressive soil chronosequences in south-western Australia. Physiol Plant 163:323–343. https://doi.org/10.1111/ppl.12704
Adams MA, Bell TL, Pate JS (2002) Phosphorus sources and availability modify growth and distribution of root clusters and nodules of native Australian legumes. Plant Cell Environ 25:837–850. https://doi.org/10.1046/j.1365-3040.2002.00867.x
Aerts R, Chapin FS (1999) The mineral nutrition of wild plants revisited: A re-evaluation of processes and patterns. Adv Ecol Res 30:1–67. https://doi.org/10.1016/S0065-2504(08)60016-1
Albornoz FE, Burgess TI, Lambers H, Etchells H, Laliberté E (2017) Native soil-borne pathogens equalize differences in competitive ability between plants of contrasting nutrient-acquisition strategies. J Ecol 105:549–557. https://doi.org/10.1111/1365-2745.12638
Albornoz FE, Dixon KW, Lambers H (2021) Revisiting mycorrhizal dogmas: are mycorrhizas really functioning as they are widely believed to do? Soil Ecol Lett 3:73–82. https://doi.org/10.1007/s42832-020-0070-2
Allsop N, Stock WD (1993) Mycorrhizal status of plants growing in the Cape Floristic Region, South Africa. Bothalia 23:91–104
Allsopp N, Colville JF, Verboom GA (2014) Fynbos: Ecology, evolution, and conservation of a megadiverse region. Oxford University Press, Oxford
Amaury de Medeiros R, Haridasan M (1985) Seasonal variations in the foliar concentrations of nutrients in some aluminium accumulating and non-accumulating species of the cerrado region of central Brazil. Plant Soil 88:433–436. https://doi.org/10.1007/BF02197499
Anderson G, Williams EG, Moir JO (1974) A comparison of the sorption of inorganic orthophosphate and inositol hexaphosphate by six acid soils. J Soil Sci 25:51–62. https://doi.org/10.1111/j.1365-2389.1974.tb01102.x
Aplin TE (1969) Poison plants of Western Australia: the toxic species of the genera Gastrolobium and Oxylobium : Champion Bay poison (G. oxylobioides Benth.), Sandplain poison (G. microcarpum Meissn.), cluster poison (G. bennettsianum C.A. Gardn.), Hutt River poison (G. propinquum C.A. Gardn.), Gilbernine poison (G. rotundifolium Meissn.). J Dept Agric W Aust 10:248–257
Barrow NJ, Debnath A, Sen A (2021) Effect of pH and prior treatment with phosphate on the rate and amount of reaction of soils with phosphate. Eur J Soil Sci 72:243–253. https://doi.org/10.1111/ejss.12968
Baxter IR, Vitek O, Lahner B, Muthukumar B, Borghi M, Morrissey J, Guerinot ML, Salt DE (2008) The leaf ionome as a multivariable system to detect a plant’s physiological status. Proc Natl Acad Sci USA 105:12081–12086
Belovitch M, Brantley S, Aubrey DP (2022) Interspecific variation in the timing and magnitude of hydraulic redistribution in a forest with distinct water sources. Plant Soil 472:451–464. https://doi.org/10.1007/s11104-021-05253-9
Berendse F, Aerts R (1987) Nitrogen-use-efficiency: a biologically meaningful definition? Funct Ecol 1:293–296
Boursier P, Läuchli A (1989) Mechanisms of chloride partitioning in the leaves of salt-stressed Sorghum bicolor L. Physiol Plant 77:537–544. https://doi.org/10.1111/j.1399-3054.1989.tb05389.x
Bowen BJ, Pate JS (1993) The significance of root starch in post-fire shoot recovery of the resprouter Stirlingia latifolia R. Br. (Proteaceae). Ann Bot 72:7–16. https://doi.org/10.1006/anbo.1993.1075
Bowen BJ, Pate JS (2004) Effect of season of burn on shoot recovery and post-fire flowering performance in the resprouter Stirlingia latifolia R. Br. (Proteaceae). Austral Ecol 29:145–155. https://doi.org/10.1111/j.1442-9993.2004.tb00307.x
Branscheid A, Sieh D, Pant BD, May P, Devers EA, Elkrog A, Schauser L, Scheible W-R, Krajinski F (2010) Expression pattern suggests a role of miR399 in the regulation of the cellular response to local Pi increase during arbuscular mycorrhizal symbiosis. Mol Plant-Microbe Interact 23:915–926. https://doi.org/10.1094/MPMI-23-7-0915
Braun-Blanquet J (1949) Übersicht der Pflanzengesellschaften Rätiens (II). Vegetatio 1:129–146
Brown G, Mitchell DT (1986) Influence of fire on the soil phosphorus status in sand plain lowland fynbos, south-western Cape. S Afr J Bot 52:67–72. https://doi.org/10.1016/S0254-6299(16)31604-0
Brundrett MC (2009) Mycorrhizal associations and other means of nutrition of vascular plants: understanding the global diversity of host plants by resolving conflicting information and developing reliable means of diagnosis. Plant Soil 320:37–77. https://doi.org/10.1007/s11104-008-9877-9
Brundrett MC, Abbott LK (1991) Roots of jarrah forest plants. I. Mycorrhizal associations of shrubs and herbaceous plants. Aust J Bot 39:445–457. https://doi.org/10.1071/BT9910445
Brundrett MC, Tedersoo L (2018) Evolutionary history of mycorrhizal symbioses and global host plant diversity. New Phytol 220:1108–1115. https://doi.org/10.1111/nph.14976
Butler OM, Elser JJ, Lewis T, Mackey B, Chen C (2018) The phosphorus-rich signature of fire in the soil–plant system: a global meta-analysis. Ecol Lett 21:335–344. https://doi.org/10.1111/ele.12896
Callaway RM (1995) Positive interactions among plants. Bot Rev 61:306–349. https://doi.org/10.1007/BF02912621
Cawthray GR, Denton MD, Grusak MA, Shane MW, Veneklaas EJ, Lambers H (2021) No evidence of regulation in root-mediated iron reduction in two Strategy I cluster-rooted Banksia species (Proteaceae). Plant Soil 203–218. https://doi.org/10.1007/s11104-021-04849-5
Chandler GT, Crisp MD, Cayzer LW, Bayer RJ (2002) Monograph of Gastrolobium (Fabaceae: Mirbelieae). Aust Syst Bot 15:619–739. https://doi.org/10.1071/SB01010
Chapin FS, Bieleski RL (1982) Mild phosphorus stress in barley and a related low-phosphorus-adapted barleygrass: phosphorus fractions and phosphate absorption in relation to growth. Physiol Plant 54:309–317. https://doi.org/10.1111/j.1399-3054.1982.tb00264.x
Chauhan R, Awasthi S, Srivastava S, Dwivedi S, Pilon-Smits EAH, Dhankher OP, Tripathi RD (2019) Understanding selenium metabolism in plants and its role as a beneficial element. Crit Rev Environ Sci Technol 49:1937–1958. https://doi.org/10.1080/10643389.2019.1598240
Chu Q, Wang X, Yang Y, Chen F, Zhang F, Feng G (2013) Mycorrhizal responsiveness of maize (Zea mays L.) genotypes as related to releasing date and available P content in soil. Mycorrhiza 23:497–505. https://doi.org/10.1007/s00572-013-0492-0
Cocks MP (1994) The ecology and nitrogen-fixing ability of selected Aspalathus spp. fynbos ecosystems. University of Cape Town, South Africa
Cocks MP, Stock WD (1997) Heat stimulated germination in relation to seed characteristics in fynbos legumes of the Western Cape Province, South Africa. S Afric J Bot 63:129–132. https://doi.org/10.1016/S0254-6299(15)30724-9
Conceição AA, Alencar TG, Souza JM, Moura ADC, Silva GA (2013) Massive post-fire flowering events in a tropical mountain region of Brazil: high episodic supply of floral resources. Acta Bot Brasil 27:847–850
Cong W-F, Suriyagoda LDB, Lambers H (2020) Tightening the phosphorus cycle through phosphorus-efficient crop genotypes. Trends Plant Sci 25:967–975. https://doi.org/10.1016/j.tplants.2020.04.013
Conn S, Gilliham M (2010) Comparative physiology of elemental distributions in plants. Ann Bot 105:1081–1102. https://doi.org/10.1093/aob/mcq027
Cook D, Lee ST, Taylor CM, Bassüner B, Riet-Correa F, Pfister JA, Gardner DR (2014) Detection of toxic monofluoroacetate in Palicourea species. Toxicon 80:9–16. https://doi.org/10.1016/j.toxicon.2013.12.003
Coskun D, Deshmukh R, Sonah H, Menzies JG, Reynolds O, Ma JF, Kronzucker HJ, Bélanger RR (2019) The controversies of silicon’s role in plant biology. New Phytol 221:67–85. https://doi.org/10.1111/nph.15343
Cowling RM, MacDonald IAW, Simmons MT (1996) The Cape Peninsula, South Africa: physiographical, biological and historical background to an extraordinary hot-spot of biodiversity. Biodivers Conserv 5:527–550
Cowling RM, Richardson DM, Mustart PJ, Richardson DM, Pierce M (1997) Fynbos. In: Cowling RM, Richardson DM, Pierce SM (eds) Vegetation of Southern Africa. Cambridge University Press, Cambridge, pp 99-130
Cramer MD, West AG, Power SC, Skelton R, Stock WD (2014) Plant ecophysiological diversity. In: Allsopp N, Colville JF, Verboom AG (eds) Fynbos: Ecology, Evolution, and Conservation of a Megadiverse Region, pp 248–273
Dayrell RLC, Arruda AJ, Pierce S, Negreiros D, Meyer PB, Lambers H, Silveira FAO (2018) Ontogenetic shifts in plant ecological strategies. Funct Ecol 32:2730–2741. https://doi.org/10.1111/1365-2435.13221
de Andrade LRM, Barros LMG, Echevarria GF, Velho do Amaral LI, Cotta MG, Rossatto DR, Haridasan M, Franco AC (2011) Al-hyperaccumulator Vochysiaceae from the Brazilian Cerrado store aluminum in their chloroplasts without apparent damage. Environ Exp Bot 70:37–42. https://doi.org/10.1016/j.envexpbot.2010.05.013
de Britto Costa P, Staudinger C, Veneklaas EJ, Oliveira RS, Lambers H (2021) Root positioning and trait shifts as dependent on a neighbour’s nutrient-acquisition strategy in severely nutrient-impoverished soils. Plant Cell Environ 44:1257–1267. https://doi.org/10.1111/pce.13991
de Tombeur F, Cornelis JT, Lambers H (2021) Silicon mobilisation by root-released carboxylates. Trends Plant Sci 26:1116–1125. https://doi.org/10.1016/j.tplants.2021.07.003
de Tombeur F, Laliberté E, Zemunik G, Faucon M-P, Cornélis J-T, Turner BL, Lambers H, Mahy G (2021) A shift from phenol to silica-based leaf defences during long-term soil and ecosystem development. Ecol Lett 24:984–995. https://doi.org/10.1111/ele.13713
Dechassa N, Schenk MK, Claassen N, Steingrobe B (2003) Phosphorus efficiency of cabbage (Brassica oleraceae L. var. capitata), carrot (Daucus carota L.), and potato (Solanum tuberosum L.). Plant Soil 250:215–224. https://doi.org/10.1023/a:1022804112388
DeGroote KV, McCartha GL, Pollard AJ (2018) Interactions of the manganese hyperaccumulator Phytolacca americana L. with soil pH and phosphate. Ecol Res 33:749–755. https://doi.org/10.1007/s11284-017-1547-z
Delgado M, Zúñiga-Feest A, Borie F, Suriyagoda L, Lambers H (2014) Divergent functioning of Proteaceae species: the South American Embothrium coccineum displays a combination of adaptive traits to survive in high-phosphorus soils. Funct Ecol 28:1356–1366. https://doi.org/10.1111/1365-2435.12303
Delhaize E, Ryan PR, Randall PJ (1993) Aluminum tolerance in wheat (Triticum aestivum L.) (II. Aluminum-stimulated excretion of malic acid from root apices). Plant Physiol 103:695–702. https://doi.org/10.1104/pp.103.3.695
Delory BM, Delaplace P, Fauconnier M-L, du Jardin P (2016) Root-emitted volatile organic compounds: can they mediate belowground plant-plant interactions? Plant Soil 402:1–26. https://doi.org/10.1007/s11104-016-2823-3
Denton MD, Veneklaas EJ, Freimoser FM, Lambers H (2007) Banksia species (Proteaceae) from severely phosphorus-impoverished soils exhibit extreme efficiency in the use and re-mobilization of phosphorus. Plant Cell Environ 30:1557–1565. https://doi.org/10.1111/j.1365-3040.2007.01733.x
Denton MD, Veneklaas EJ, Lambers H (2007) Does phenotypic plasticity in carboxylate exudation differ among rare and widespread Banksia species (Proteaceae)? New Phytol 173:592–599. https://doi.org/10.1111/j.1469-8137.2006.01956.x
Dietz KJ, Schramm M, Lang B, Lanzl-Schramm A, Dürr C, Martinoia E (1992) Characterization of the epidermis from barley primary leaves. Planta 187:431–437. https://doi.org/10.1007/BF00199960
Doolette AL, Smernik RJ, Dougherty WJ (2011) A quantitative assessment of phosphorus forms in some Australian soils. Soil Res 49:152–165. https://doi.org/10.1071/SR10092
Dowling A, O Sadras V, Roberts P, Doolette A, Zhou Y, Denton MD (2021) Legume-oilseed intercropping in mechanised broadacre agriculture – a review. Field Crops Res 260:107980. https://doi.org/10.1016/j.fcr.2020.107980
Earl KD, Syers JK, McLaughlin JR (1979) Origin of the effects of citrate, tartrate, and acetate on phosphate sorption by soils and synthetic gels. Soil Sci Soc Am J 43:674–678. https://doi.org/10.2136/sssaj1979.03615995004300040009x
Fageria NK, Santos AB, Barbosa Filho MP, Guimarães CM (2008) Iron toxicity in lowland rice. J Plant Nutr 31:1676–1697. https://doi.org/10.1080/01904160802244902
Fidelis A, Rosalem P, Zanzarini V, Camargos LS, Martins AR (2019) From ashes to flowers: a savanna sedge initiates flowers 24 h after fire. Ecology 100:e02648. https://doi.org/10.1002/ecy.2648
Fidelis A, Zirondi HL (2021) And after fire, the Cerrado flowers: a review of post-fire flowering in a tropical savanna. Flora 280:151849. https://doi.org/10.1016/j.flora.2021.151849
Flematti GR, Dixon KW, Smith SM (2015) What are karrikins and how were they ‘discovered’ by plants? BMC Biol 13. https://doi.org/10.1186/s12915-015-0219-0
Flematti GR, Ghisalberti EL, Dixon KW, Trengove RD (2004) Molecular weight of a germination-enhancing compound in smoke. Plant Soil 263:1–4. https://doi.org/10.1023/B:PLSO.0000047804.82920.4f
Fletcher AL, Kirkegaard JA, Peoples MB, Robertson MJ, Whish J, Swan AD (2016) Prospects to utilise intercrops and crop variety mixtures in mechanised, rain-fed, temperate cropping systems. Crop Past Sci 67:1252–1267. https://doi.org/10.1071/CP16211
Föhse D, Claassen N, Jungk A (1991) Phosphorus efficiency of plants. II. Significance of root radius, root hairs and cation-anion balance for phosphorus influx in seven plant species. Plant Soil 132:261–272. https://doi.org/10.1007/BF00010407
Gao J, Wang F, Ranathunge K, Arruda AJ, Cawthray GR, Clode PL, He X, Leopold M, Roessner U, Rupasinghe T, Zhong H, Lambers H (2020) Edaphic niche characterization of four Proteaceae reveals unique calcicole physiology linked to hyper-endemism of Grevillea thelemanniana. New Phytol 228:869–883. https://doi.org/10.1111/nph.16833
Gardner WK, Parbery DG, Barber DA (1981) Proteoid root morphology and function in Lupinus albus Plant Soil 60:143–147. https://doi.org/10.1007/BF02374894
Gattullo CE, Allegretta I, Medici L, Fijan R, Pii Y, Cesco S, Mimmo T, Terzano R (2016) Silicon dynamics in the rhizosphere: connections with iron mobilization. J Plant Nutr Soil Sci 179:409–417. https://doi.org/10.1002/jpln.201500535
Geelhoed JS, Hiemstra T, Van Riemsdijk WH (1998) Competitive interaction between phosphate and citrate on goethite. Environ Sci Technol 32:2119–2123. https://doi.org/10.1021/es970908y
Giardina CP, Sanford RL, Døckersmith IC, Jaramillo VJ (2000) The effects of slash burning on ecosystem nutrients during the land preparation phase of shifting cultivation. Plant Soil 220:247–260. https://doi.org/10.1023/A:1004741125636
Giles CD, George TS, Brown LK, Mezeli MM, Richardson AE, Shand CA, Wendler R, Darch T, Menezes-Blackburn D, Cooper P, Stutter MI, Lumsdon DG, Blackwell MSA, Wearing C, Zhang H, Haygarth PM (2017) Does the combination of citrate and phytase exudation in Nicotiana tabacum promote the acquisition of endogenous soil organic phosphorus? Plant Soil 412:43–59. https://doi.org/10.1007/s11104-016-2884-3
Grime JP (1977) Evidence for the existence of three primary strategies in plants and its relevance to ecological and evolutionary theory. Am Nat 111:1169–1194. https://doi.org/10.1086/283244
Grove TS, O’connell A M, Malajczuk N (1980) Effects of fire on the growth, nutrient content and rate of nitrogen fixation of the cycad Macrozamia riedlei Aust J Bot 28:271–281. https://doi.org/10.1071/BT9800271
Guilherme Pereira C, Clode PL, Oliveira RS, Lambers H (2018) Eudicots from severely phosphorus-impoverished environments preferentially allocate phosphorus to their mesophyll. New Phytol 218:959–973. https://doi.org/10.1111/nph.15043
Guilherme Pereira C, Hayes PE, O’Sullivan O, Weerasinghe L, Clode PL, Atkin OK, Lambers H (2019) Trait convergence in photosynthetic nutrient-use efficiency along a 2-million year dune chronosequence in a global biodiversity hotspot. J Ecol 107:2006–2023. https://doi.org/10.1111/1365-2745.13158
Güsewell S (2017) Regulation of dauciform root formation and root phosphatase activities of sedges (Carex) by nitrogen and phosphorus. Plant Soil 415:57–72. https://doi.org/10.1007/s11104-016-3142-4
Güsewell S, Schroth MH (2017) How functional is a trait? Phosphorus mobilization through root exudates differs little between Carex species with and without specialized dauciform roots. New Phytol 215:1438–1450. https://doi.org/10.1111/nph.14674
Hansen A, Pate JS, Hansen AP (1991) Growth and reproductive performance of a seeder and a resprouter species of Bossiaea as a function of plant age after fire. Ann Bot 67:497–509. https://doi.org/10.1093/oxfordjournals.aob.a088190
Haridasan M, De Araújo GM (1988) Aluminium-accumulating species in two forest communities in the cerrado region of central Brazil. For Ecol Manag 24:15–26. https://doi.org/10.1016/0378-1127(88)90021-7
Hawkins H-J, Hettasch H, Mesjasz-Przybylowicz J, Przybylowicz W, Cramer MD (2008) Phosphorus toxicity in the Proteaceae: a problem in post-agricultural lands. Sci Hort 117:357–365. https://doi.org/10.1016/j.scienta.2008.05.001
Hayes P, Turner BL, Lambers H, Laliberté E (2014) Foliar nutrient concentrations and resorption efficiency in plants of contrasting nutrient-acquisition strategies along a 2-million-year dune chronosequence. J Ecol 102:396–410. https://doi.org/10.1111/1365-2745.12196
Hayes PE, Adem GD, Pariasca-Tanaka J, Wissuwa M (2022) Leaf phosphorus fractionation in rice to understand internal phosphorus-use efficiency. Ann Bot 129:287–302. https://doi.org/10.1093/aob/mcab138
Hayes PE, Clode PL, Oliveira RS, Lambers H (2018) Proteaceae from phosphorus-impoverished habitats preferentially allocate phosphorus to photosynthetic cells: an adaptation improving phosphorus-use efficiency. Plant Cell Environ 41:605–619. https://doi.org/10.1111/pce.13124
Heddle EM, Specht RL (1975) Dark Island Heath (Ninety-Mile Plain, South Australia). VIII. The effect of fertilizers on composition and growth, 1950–1972. Aust J Bot 23:151–164. https://doi.org/10.1071/BT9750151
Hester AJ, Hobbs RJ (1992) Influence of fire and soil nutrients on native and non-native annuals at remnant vegetation edges in the Western Australian wheatbelt. J Veg Sci 3:101–108. https://doi.org/10.2307/3236003
Hodson MJ, Evans DE (1995) Aluminium/silicon interactions in higher plants. J Exp Bot 46:161–171. https://doi.org/10.1093/jxb/46.2.161
Homulle Z, George TS, Karley AJ (2022) Root traits with team benefits: understanding belowground interactions in intercropping systems. Plant Soil 471:1–26. https://doi.org/10.1007/s11104-021-05165-8
Hopper SD (2009) OCBIL theory: towards an integrated understanding of the evolution, ecology and conservation of biodiversity on old, climatically buffered, infertile landscapes. Plant Soil 322:49–86. https://doi.org/10.1007/s11104-009-0068-0
Hopper SD, Lambers H, Silveira F, A O, Fiedler PL (2021) OCBIL theory examined: reassessing evolution, ecology and conservation in the world’s ancient, climatically buffered and infertile landscapes. Bot J Linn Soc 133:266–296. https://doi.org/10.1093/biolinnean/blaa213
Hou E, Luo Y, Kuang Y, Chen C, Lu X, Jiang L, Luo X, Wen D (2020) Global meta-analysis shows pervasive phosphorus limitation of aboveground plant production in natural terrestrial ecosystems. Nat Comm 11:637. https://doi.org/10.1038/s41467-020-14492-w
Houlton BZ, Wang Y-P, Vitousek PM, Field CB (2008) A unifying framework for dinitrogen fixation in the terrestrial biosphere. Nature 454:327–330. https://doi.org/10.1038/nature07028
Huang G, Hayes PE, Ryan MH, Pang J, Lambers H (2017) Peppermint trees shift their phosphorus-acquisition strategy along a strong gradient of plant-available phosphorus by increasing their transpiration. Oecologia 185:487–400. https://doi.org/10.1007/s00442-017-3961-x
Hurd T, Schwintzer C (1996) Formation of cluster roots in Alnus incana ssp. rugosa and other Alnus species. Can J Bot 74:684–1686
Husby C (2013) Biology and functional ecology of Equisetum with emphasis on the giant horsetails. Bot Rev 79:147–177. https://doi.org/10.1007/s12229-012-9113-4
Jeong J, Guerinot ML (2009) Homing in on iron homeostasis in plants. Trends Plant Sci 14:280–285. https://doi.org/10.1016/j.tplants.2009.02.006
Jeong K, Julia CC, Waters DLE, Pantoja O, Wissuwa M, Heuer S, Liu L, Rose TJ (2017) Remobilisation of phosphorus fractions in rice flag leaves during grain filling: Implications for photosynthesis and grain yields. PLoS ONE 12:e0187521. https://doi.org/10.1371/journal.pone.0187521
Karley AJ, Leigh RA, Sanders D (2000) Differential ion accumulation and ion fluxes in the mesophyll and epidermis of barley. Plant Physiol 122:835–844. https://doi.org/10.1104/pp.122.3.835
Kidd DR, Ryan MH, Hahne D, Haling RE, Lambers H, Sandral GA, Simpson RJ, Cawthray GR (2018) The carboxylate composition of rhizosheath and root exudates from twelve species of grassland and crop legumes with special reference to the occurrence of citramalate. Plant Soil 424:389–403. https://doi.org/10.1007/s11104-017-3534-0
Killingbeck KT (1996) Nutrients in senesced leaves: keys to the search for potential resorption and resorption proficiency. Ecology 77:1716–1727. https://doi.org/10.2307/2265777
Kong C-H, Zhang S-Z, Li Y-H, Xia Z-C, Yang X-F, Meiners SJ, Wang P (2018) Plant neighbor detection and allelochemical response are driven by root-secreted signaling chemicals. Nat Comm 9:3867. https://doi.org/10.1038/s41467-018-06429-1
Korczynskyj D, Lamont BB (2005) Grasstree (Xanthorrhoea preissii) recovery after fire in two seasons and habitats. Aust J Bot 53:509–515. https://doi.org/10.1071/BT05006
Kozovits AR, Bustamante MMC, Garofalo CR, Bucci S, Franco AC, Goldstein G, Meinzer FC (2007) Nutrient resorption and patterns of litter production and decomposition in a Neotropical Savanna. Funct Ecol 21:1034–1043. https://doi.org/10.1111/j.1365-2435.2007.01325.x
Krebs HC, Kemmerling W, Habermehl G (1994) Qualitative and quantitative determination of fluoroacetic acid in Arrabidea bilabiata and Palicourea marcgravii by 19F-NMR spectroscopy. Toxicon 32:909–913. https://doi.org/10.1016/0041-0101(94)90369-7
Kuppusamy T, Giavalisco P, Arvidsson S, Sulpice R, Stitt M, Finnegan PM, Scheible W-R, Lambers H, Jost R (2014) Lipid biosynthesis and protein concentration respond uniquely to phosphate supply during leaf development in highly phosphorus-efficient Hakea prostrata Plant Physiol 166:1891–1911
Kutiel P, Shaviv A (1989) Effect of simulated forest fire on the availability of N and P in mediterranean soils. Plant Soil 120:57–63. https://doi.org/10.1007/BF02370290
Laliberté E, Turner BL, Costes T, Pearse SJ, Wyrwoll K-H, Zemunik G, Lambers H (2012) Experimental assessment of nutrient limitation along a 2-million year dune chronosequence in the south-western Australia biodiversity hotspot. J Ecol 100:631–642. https://doi.org/10.1111/j.1365-2745.2012.01962.x
Lambers HEd (2014) Plant life on the sandplains in Southwest Australia, a global biodiversity hotspot. University of Western Australia Publishing, Crawley, Australia
Lambers H (2022) Phosphorus acquisition and utilization in plants. Annu Rev Plant Biol 73, in press. https://doi.org/10.1146/annurev-arplant-102720-125738
Lambers H, Ahmedi I, Berkowitz O, Dunne C, Finnegan PM, Hardy GESJ, Jost R, Laliberté E, Pearse SJ, Teste FP (2013) Phosphorus nutrition of phosphorus-sensitive Australian native plants: threats to plant communities in a global biodiversity hotspot. Conserv Physiol 1. https://doi.org/10.1093/conphys/cot1010
Lambers H, Albornoz F, Kotula L, Laliberté E, Ranathunge K, Teste FP, Zemunik G (2018) How belowground interactions contribute to the coexistence of mycorrhizal and non-mycorrhizal species in severely phosphorus-impoverished hyperdiverse ecosystems. Plant Soil 424:11–34. https://doi.org/10.1007/s11104-017-3427-2
Lambers H, Albornoz FE, Arruda AJ, Barker T, Finnegan PM, Gille C, Gooding H, Png GK, Ranathunge K, Zhong H 2019 Nutrient-acquisition strategies. In: Lambers H (ed) A jewel in the crown of a global biodiversity hotspot. Kwongan Foundation and the Western Australian Naturalists’ Club Inc, Perth, pp 227–248
Lambers H, Bishop JG, Hopper SD, Laliberté E, Zúñiga-Feest A (2012) Phosphorus-mobilization ecosystem engineering: the roles of cluster roots and carboxylate exudation in young P-limited ecosystems. Ann Bot 110:329–348. https://doi.org/10.1093/aob/mcs130
Lambers H, Cawthray GR, Giavalisco P, Kuo J, Laliberté E, Pearse SJ, Scheible W-R, Stitt M, Teste F, Turner BL (2012) Proteaceae from severely phosphorus-impoverished soils extensively replace phospholipids with galactolipids and sulfolipids during leaf development to achieve a high photosynthetic phosphorus-use-efficiency. New Phytol 196:1098–1108. https://doi.org/10.1111/j.1469-8137.2012.04285.x
Lambers H, Clode PL, Hawkins H-J, Laliberté E, Oliveira RS, Reddell P, Shane MW, Stitt M, Weston P (2015) Metabolic adaptations of the non-mycotrophic Proteaceae to soil with a low phosphorus availability. Plaxton WC, Lambers H (eds) Annual Plant Reviews, vol 48, Phosphorus Metabolism in Plants. Wiley, Chicester, pp 289–336
Lambers H, Guilherme Pereira C, Wright IJ, Bellingham PJ, Bentley LP, Boonman A, Cernusak LA, Foulds W, Gleason SM, Gray EM, Hayes PE, Kooyman RM, Malhi Y, Richardson SJ, Shane MW, Staudinger C, Stock WD, Swarts NG, Turner BL, Turner J, Veneklaas EJ, Wasaki J, Westoby M, Xu Y (2021) Leaf manganese concentrations as a tool to assess belowground plant functioning in phosphorus-impoverished environments. Plant Soil 461:43–61. https://doi.org/10.1007/s11104-020-04690-2
Lambers H, Hayes PE, Laliberté E, Oliveira RS, Turner BL (2015) Leaf manganese accumulation and phosphorus-acquisition efficiency. Trends Plant Sci 20:83–90. https://doi.org/10.1016/j.tplants.2014.10.007
Lambers H, Oliveira RS (2019) Plant physiological ecology, 3rd edn. Springer, Cham
Lambers H, Raven JA, Shaver GR, Smith SE (2008) Plant nutrient-acquisition strategies change with soil age. Trends Ecol Evol 23:95–103. https://doi.org/10.1016/j.tree.2007.10.008
Lambers H, Shane MW, Laliberté E, Swarts ND, Teste FP, Zemunik G (2014) Plant mineral nutrition. In: Lambers H (ed) Plant Life on the Sandplains in Southwest Australia, a Global Biodiversity Hotspot. UWA Publishing, Crawley, pp 101–127
Lamont B (1982) Mechanisms for enhancing nutrient uptake in plants, with particular reference to Mediterranean South Africa and Western Australia. Bot Rev 48:597–689. https://doi.org/10.1007/BF02860714
Lamont BB (1972) ’Proteoid’ roots in the legume Viminaria juncea Search 3:90–91
Lamont BB, Downes KS (2011) Fire-stimulated flowering among resprouters and geophytes in Australia and South Africa. Plant Ecol 212:2111–2125. https://doi.org/10.1007/s11258-011-9987-y
Lamont BB, Downes S (1979) The longevity, flowering and fire history of the grasstrees Xanthorrhoea preissii and Kingia australis J Appl Ecol 16:893–899. https://doi.org/10.2307/2402862
Lan ZM, Lin XJ, Zhang WG, Zhang H, Wu YQ (2012) Effect of P deficiency on the emergence of Astragalus L. root exudates and mobilization of sparingly soluble phosphorus. Sci Agric Sin 45:1521–1531. https://doi.org/10.3864/j.issn.0578-1752.2012.08.008
Le Stradic S, Roumet C, Durigan G, Cancian L, Fidelis A (2021) Variation in biomass allocation and root functional parameters in response to fire history in Brazilian savannas. J Ecol 109:4143–4157. https://doi.org/10.1111/1365-2745.13786
Lee ST, Cook D, Riet-Correa F, Pfister JA, Anderson WR, Lima FG, Gardner DR (2012) Detection of monofluoroacetate in Palicourea and Amorimia species. Toxicon 60:791–796. https://doi.org/10.1016/j.toxicon.2012.05.029
Leiser WL, Rattunde HF, Weltzien E, Cisse N, Abdou M, Diallo A, Tourè AO, Magalhaes JV, Haussmann BI (2014) Two in one sweep: aluminum tolerance and grain yield in P-limited soils are associated to the same genomic region in West African Sorghum. BMC Plant Biol 14. https://doi.org/10.1186/s12870-014-0206-6
Leitch CJ, Flinn DW, van de Graaff RHM (1983) Erosion and nutrient loss resulting from Ash Wednesday (February 1983) wildfires a case study. Aust For 46:173–180. https://doi.org/10.1080/00049158.1983.10674396
Li H, Zhang F, Rengel Z, Shen J (2013) Rhizosphere properties in monocropping and intercropping systems between faba bean (Vicia faba L.) and maize (Zea mays L.) grown in a calcareous soil. Crop Past Sci 64:976–984. https://doi.org/10.1071/CP13268
Li J-T, Gurajala HK, Wu L-H, Van der Ent A, Qiu R-l, Baker AJM, Tang Y-T, Yang X-E, Shu W-S (2018) Hyperaccumulator plants from China: a synthesis of the current state of knowledge. Environ Sci Technol 52:11980–11994. https://doi.org/10.1021/acs.est.8b01060
Li L, Tilman D, Lambers H, Zhang F (2014) Plant diversity and overyielding: insights from belowground facilitation of intercropping in agriculture. New Phytol 203:63–69. https://doi.org/10.1111/nph.12778
Li W, Finnegan PM, Dai Q, Guo D, Yang M (2021) Metabolic acclimation supports higher aluminium-induced secretion of citrate and malate in an aluminium-tolerant hybrid clone of Eucalyptus. BMC Plant Biol 21:14. https://doi.org/10.1186/s12870-020-02788-4
Li X, Yang Z, Zhang Y, n, Yu L, Ding C, Liao Y, Dai C, Wang X (2020) Atractylodes lancea volatiles induce physiological responses in neighboring peanut plant during intercropping. Plant Soil 453:409–422. https://doi.org/10.1007/s11104-020-04615-z
Lim TK (2012) Anacardium occidentale. In: Lim TK (ed) Edible Medicinal and Non-Medicinal Plants: vol 1, Fruits. Springer Netherlands, Dordrecht, pp 45–68
Liu C, Liu W-S, van der Ent A, Morel JL, Zheng H-X, Wang G-B, Tang Y-T, Qiu R-L (2021) Simultaneous hyperaccumulation of rare earth elements, manganese and aluminum in Phytolacca americana in response to soil properties. Chemosphere 131096. https://doi.org/10.1016/j.chemosphere.2021.131096
Lopez-Hernandez D, Flores D, Siegert G, Rodriguez JV (1979) The effect of some organic anions on phosphate removal from acid and calcareous soils. Soil Sci 128:312–326
Louis I, Racette S, Torrey JG (1990) Occurrence of cluster roots on Myrica cerifera L. (Myricaceae) in water culture in relation to phosphorus nutrition. New Phytol 115:311–317
Lu M, Bond WJ, Sheffer E, Cramer MD, West AG, Allsopp N, February EC, Chimphango S, Ma Z, Slingsby JA, Hedin LO (2022) Biome boundary maintained by intense belowground resource competition in world’s thinnest-rooted plant community. Proc Natl Acad Sci USA 119:e2117514119. https://doi.org/10.1073/pnas.2117514119
Lux A, Luxová M, Abe J, Morita S, Inanaga S (2003) Silicification of bamboo (Phyllostachys heterocycla Mitf.) root and leaf. Plant Soil 255:85–91. https://doi.org/10.1023/A:1026157424794
Ma JF (2005) Plant root responses to three abundant soil minerals: silicon, aluminum and iron. Crit Rev Plant Sci 24:267–281. https://doi.org/10.1080/07352680500196017
MacAlister D, Muasya AM, Chimphango SBM (2018) Linking root traits to superior phosphorus uptake and utilisation efficiency in three Fabales in the Core Cape Subregion, South Africa. Funct Plant Biol 45:760–770. https://doi.org/10.1071/FP17209
Magalhaes JV, Liu J, Guimaraes CT, Lana UGP, Alves VMC, Wang Y-H, Schaffert RE, Hoekenga OA, Pineros MA, Shaff JE, Klein PE, Carneiro NP, Coelho CM, Trick HN, Kochian LV (2007) A gene in the multidrug and toxic compound extrusion (MATE) family confers aluminum tolerance in sorghum. Nat Gen 39:1156–1161. https://doi.org/10.1038/ng2074
Mamode Cassim A, Gouguet P, Gronnier J, Laurent N, Germain V, Grison M, Boutté Y, Gerbeau-Pissot P, Simon-Plas F, Mongrand S (2019) Plant lipids: key players of plasma membrane organization and function. Prog Lipid Res 73:1–27. https://doi.org/10.1016/j.plipres.2018.11.002
Marsh AS, Arnone JA, Bormann BT, Gordon JC (2000) The role of Equisetum in nutrient cycling in an Alaskan shrub wetland. J Ecol 88:999–1011. https://doi.org/10.1046/j.1365-2745.2000.00520.x
Masuda G, Maruyama H, Lambers H, Wasaki J (2021) Formation of dauciform roots by Japanese native Cyperaceae and their contribution to phosphorus dynamics in soils. Plant Soil 461:107–118. https://doi.org/10.1007/s11104-020-04565-6
McArthur RH, Wilson EO (1967) The theory of island biogeography. Princeton University Press, Princeton
McArthur WM (1991) Reference soils of South-western Australia. Department of Agriculture Western Australia, South Perth
Miller BP, Dixon KW (2014) Plants and fire in kwongan vegetation. In: Lambers H (ed) Plant Life on the Sandplains in Southwest Australia, a Global Biodiversity Hotspot. UWA Publishing, Crawley, pp 147–169
Muler AL, Oliveira RS, Lambers H, Veneklaas EJ (2014) Does cluster-root activity of Banksia attenuata (Proteaceae) benefit phosphorus or micronutrient uptake and growth of neighbouring shrubs? Oecologia 174:23–31. https://doi.org/10.1007/s00442-013-2747-z
Myers N, Mittermeier RA, Mittermeier CG, da Fonseca GA, Kent J (2000) Biodiversity hotspots for conservation priorities. Nature 403:853–858. https://doi.org/10.1038/35002501
Nagarajah S, Posner AM, Quirk JP (1970) Competitive adsorption of phosphate with polygalacturonate and other organic anions on kaolinite and oxide surfaces. Nature 228:83–85. https://doi.org/10.1038/228083a0
Nakamura Y (2017) Plant phospholipid diversity: emerging functions in metabolism and protein–lipid interactions. Trends Plant Sci 22:1027–1040. https://doi.org/10.1016/j.tplants.2017.09.002
Neumann G, Römheld V (1999) Root excretion of carboxylic acids and protons in phosphorus-deficient plants. Plant Soil 211:121–130. https://doi.org/10.1023/a:1004380832118
Nge FJ, Cambridge ML, Ellsworth DS, Zhong H, Lambers H (2020) Cluster roots are common in Daviesia and allies (Mirbelioids; Fabaceae). J R Soc W Austr 103:111–118
Nichols DG, Jones DL, Beardsell DV (1979) The effect of phosphorus on the growth of Grevillea `Poorinda firebird’ in soil-less potting-mixtures. Sci Hort 11:197–205. https://doi.org/10.1016/0304-4238(79)90045-1
Olde Venterink H (2011) Legumes have a higher root phosphatase activity than other forbs, particularly under low inorganic P and N supply. Plant Soil 347:137–146. https://doi.org/10.1007/s11104-011-0834-7
Oliveira RS (2004) Comparative water use and water acquisition strategies of trees from the Brazilian Cerrado and Amazônia. University of California, Berkeley
Oliveira RS, Abrahão A, Pereira C, Teodoro GS, Brum M, Alcantara S, Lambers H 2016 Ecophysiology of campos rupestres plants. In: Fernandes WG (ed) Ecology and Conservation of Mountaintop Grasslands in Brazil. Springer International Publishing, Cham, pp 227–272
Oliveira RS, Batista JAN, Proença CE, B, Bianchetti LB (1996) Influência do fogo na floração de espécies de Orchidaceae em cerrado. Anais do Simpósio Impacto das Queimadas sobre os Ecossistemas e Mudanças Globais 3o Congresso de Ecologia do Brasil, Brasília, pp 61–67
Oliveira RS, Galvão HC, de Campos MCR, Eller CB, Pearse SJ, Lambers H (2015) Mineral nutrition of campos rupestres plant species on contrasting nutrient-impoverished soil types. New Phytol 205:1183–1194. https://doi.org/10.1111/nph.13175
Oliveras I, Meirelles ST, Hirakuri VL, Freitas CR, Miranda HS, Pivello VR (2013) Effects of fire regimes on herbaceous biomass and nutrient dynamics in the Brazilian savanna. Int J Wildland Fire 22:368–380. https://doi.org/10.1071/WF10136
Orians GH, Milewski AV (2007) Ecology of Australia: the effects of nutrient-poor soils and intense fires. Biol Rev 82:393–423
Pang J, Ruchi B, Zhao H, Bansal R, Bohuon E, Lambers H, Ryan MH, Ranathunge K, Siddique KMH (2018) The carboxylate-releasing phosphorus-mobilising strategy could be proxied by foliar manganese concentration in a large set of chickpea germplasm under low phosphorus supply. New Phytol 219:518–529. https://doi.org/10.1111/nph.15200
Pang J, Ryan MH, Tibbett M, Cawthray GR, Siddique KHM, Bolland MDA, Denton MD, Lambers H (2010) Variation in morphological and physiological parameters in herbaceous perennial legumes in response to phosphorus supply. Plant Soil 331:241–255. https://doi.org/10.1007/s11104-009-0249-x
Pang J, Wang Y, Lambers H, Tibbett M, Siddique KHM, Ryan MH (2013) Commensalism in an agroecosystem: hydraulic redistribution by deep-rooted legumes improves survival of a droughted shallow-rooted legume companion. Physiol Plant 149:79–90. https://doi.org/10.1111/ppl.12020
Pant BD, Buhtz A, Kehr J, Scheible W-R (2008) MicroRNA399 is a long-distance signal for the regulation of plant phosphate homeostasis. Plant J 53:731–738. https://doi.org/10.1111/j.1365-313X.2007.03363.x
Parfitt RL (1979) The availability of P from phosphate-goethite bridging complexes. Desorption and uptake by ryegrass. Plant Soil 53:55–65. https://doi.org/10.1007/BF02181879
Parry GD (1981) The meanings of r- and K-selection. Oecologia 48:260–264. https://doi.org/10.1007/BF00347974
Pasta PC, Durigan G, Moraes ICF, Ribeiro LF, Haminiuk CWI, Branco IG (2019) Physicochemical properties, antioxidant potential and mineral content of Miconia albicans (Sw.) Triana: a fruit with high aluminium content. Brazilian J Bot 42:209–216. https://doi.org/10.1007/s40415-019-00532-3
Pate JS, Casson NE, Rullo J, Kuo J (1985) Biology of fire ephemerals of the sandplains of the kwongan of south-western Australia. Funct Plant Biol 12:641–655. https://doi.org/10.1071/PP9850641
Pate JS, Froend RH, Bowen BJ, Hansen A, Kuo J (1990) Seedling growth and storage characteristics of seeder and resprouter species of Mediterranean-type ecosystems of S. W. Australia. Ann Bot 65:585–601. https://doi.org/10.1093/oxfordjournals.aob.a087976
Pate JS, Meney KA, Dixon KW (1991) Contrasting growth and morphological characteristics of fire-sensitive (obligate seeder) and fire-resistant (resprouter) species of Restionaceae (S Hemisphere Restiads) from south-western Western-Australia. Aust J Bot 39:505–525. https://doi.org/10.1071/BT9910505
Peñuelas J, Asensio D, Tholl D, Wenke K, Rosenkranz M, Piechulla B, Schnitzler JP (2014) Biogenic volatile emissions from the soil. Plant Cell Environ 37:1866–1891. https://doi.org/10.1111/pce.12340
Peters RA, Hall RJ, Ward PFV, Sheppard N (1960) The chemical nature of the toxic compounds containing fluorine in the seeds of Dichapetalum toxicarium Biochem J 77:17–22. https://doi.org/10.1042/bj0770017
Pinto Irish K, Harvey M-A, Erskine PD, van der Ent A (2021) Root foraging and selenium uptake in the Australian hyperaccumulator Neptunia amplexicaulis and non-accumulator Neptunia gracilis Plant Soil 462:219–233. https://doi.org/10.1007/s11104-021-04843-x
Png GK, Turner BL, Albornoz FE, Hayes PE, Lambers H, Laliberté E (2017) Greater root phosphatase activity in nitrogen-fixing rhizobial but not actinorhizal plants with declining phosphorus availability. J Ecol 105:1246–1255. https://doi.org/10.1111/1365-2745.12758
Porder S, Ramachandran S (2013) The phosphorus concentration of common rocks—a potential driver of ecosystem P status. Plant Soil 367:41–55. https://doi.org/10.1007/s11104-012-1490-2
Power S, Cramer M, Verboom G, Chimphango S (2011) Legume seeders of the Cape Floristic Region inhabit more fertile soils than congeneric resprouters—sometimes. Plant Ecol 212:1979–1989. https://doi.org/10.1007/s11258-011-9958-3
Power SC, Cramer MD, Verboom GA, Chimphango SBM (2010) Does phosphate acquisition constrain legume persistence in the fynbos of the Cape Floristic Region? Plant Soil 334:33–46. https://doi.org/10.1007/s11104-010-0311-8
Purnell HM (1960) Studies of the family Proteaceae. I. Anatomy and morphology of the roots of some Victorian species. Aust J Bot 8:38–50. https://doi.org/10.1071/BT9600038
Raison RJ, Khanna PK, Woods PV (1985) Mechanisms of element transfer to the atmosphere during vegetation fires. Can J For Res 15:132–140. https://doi.org/10.1139/x85-022
Raven JA, Lambers H, Smith SE, Westoby M (2018) Costs of acquiring phosphorus by vascular land plants: patterns and implications for plant coexistence. New Phytol 217:1420–1427. https://doi.org/10.1111/nph.14967
Reddell P, Yun Y, Shipton WA (1997) Cluster roots and mycorrhizae in Casuarina cunninghamiana: their occurrence and formation in relation to phosphorus supply. Aust J Bot 45:41–51. https://doi.org/10.1071/BT96049
Reichert T, Rammig A, Fuchslueger L, Lugli LF, Quesada CA, Fleischer K (2022) Plant phosphorus-use and -acquisition strategies in Amazonia. New Phytol. https://doi.org/10.1111/nph.17985
Resende JCF, Markewitz D, Klink CA, Bustamante MM, d C, Davidson EA (2011) Phosphorus cycling in a small watershed in the Brazilian Cerrado: impacts of frequent burning. Biogeochemistry 105:105–118. https://doi.org/10.1007/s10533-010-9531-5
Römheld V, Schaaf G (2004) Iron transport in plants: future research in view of a plant nutritionist and a molecular biologist. Soil Sci Plant Nutr 50:1003–1012. https://doi.org/10.1080/00380768.2004.10408567
Rudall PJ, Conran JG (2012) Systematic placement of Dasypogonaceae among commelinid monocots: evidence from flowers and fruits. Bot Rev 78:398–415. https://doi.org/10.1007/s12229-012-9103-6
Rutherford MC, Powrie LW, Husted LB, Turner RC (2011) Early post-fire plant succession in Peninsula Sandstone Fynbos: the first three years after disturbance. S Afric J Bot 77:665–674. https://doi.org/10.1016/j.sajb.2011.02.002
Ryan MH, Tibbett M, Edmonds-Tibbett T, Suriyagoda LDB, Lambers H, Cawthray GR, Pang J (2012) Carbon trading for phosphorus gain: the balance between rhizosphere carboxylates and mycorrhizal symbiosis in plant phosphorus acquisition. Plant Cell Environ 35:2061–2220. https://doi.org/10.1111/j.1365-3040.2012.02547.x
Sandral GA, Price A, Hildebrand SM, Fuller CG, Haling RE, Stefanski A, Yang Z, Culvenor RA, Ryan MH, Kidd DR, Diffey S, Lambers H, Simpson RJ (2019) Field benchmarking of the critical external phosphorus requirements of pasture legumes for southern Australia. Crop Past Sci 70:1080–1096. https://doi.org/10.1071/CP19014
Schaller J, Tischer A, Struyf E, Bremer M, Belmonte DU, Potthast K (2015) Fire enhances phosphorus availability in topsoils depending on binding properties. Ecology 96:1598–1606. https://doi.org/10.1890/14-1311.1
Selivanov IA, Utemova LD (1969) Root anatomy of sedges in relation to their mycotrophy (in Russian). Trans Perm State Pedag Inst 68:45–55
Severne BC, Brooks RR (1972) A nickel-accumulating plant from Western Australia. Planta 103:91–94. https://doi.org/10.1007/bf00394610
Shane MW, Cawthray GR, Cramer MD, Kuo J, Lambers H (2006) Specialized ‘dauciform’ roots of Cyperaceae are structurally distinct, but functionally analogous with ‘cluster’ roots. Plant Cell Environ 29:1989–1999. https://doi.org/10.1111/j.1365-3040.2006.01574.x
Shane MW, Cramer MD, Funayama-Noguchi S, Cawthray GR, Millar AH, Day DA, Lambers H (2004) Developmental physiology of cluster-root carboxylate synthesis and exudation in harsh hakea. Expression of phosphoenolpyruvate carboxylase and the alternative oxidase. Plant Physiol 135:549–560
Shane MW, Lambers H (2005) Cluster roots: a curiosity in context. Plant Soil 274:101–125. https://doi.org/10.1007/s11104-004-2725-7
Shane MW, McCully ME, Lambers H (2004) Tissue and cellular phosphorus storage during development of phosphorus toxicity in Hakea prostrata (Proteaceae). J Exp Bot 55:1033–1044
Silva VM, Rimoldi Tavanti RF, Gratão PL, Alcock TD, Reis AR (2020) d Selenate and selenite affect photosynthetic pigments and ROS scavenging throughistinct mechanisms in cowpea (Vigna unguiculata (L.) walp) plants. Ecotoxicol Environ Saf 201:110777. https://doi.org/10.1016/j.ecoenv.2020.110777
Silveira FO, Negreiros D, Barbosa NU, Buisson E, Carmo F, Carstensen D, Conceição A, Cornelissen T, Echternacht L, Fernandes GW, Garcia Q, Guerra T, Jacobi C, Lemos-Filho J, Le Stradic S, Morellato L, Neves F, Oliveira R, Schaefer C, Viana P, Lambers H (2016) Ecology and evolution of plant diversity in the endangered campo rupestre: a neglected conservation priority. Plant Soil 403:129–152. https://doi.org/10.1007/s11104-015-2637-8
Simon MF, Grether R, De Queiroz LP, Skema C, Pennington RT, Hughes CE (2009) Recent assembly of the Cerrado, a neotropical plant diversity hotspot, by in situ evolution of adaptations to fire. Proc Natl Acad Sci USA 106:20359–20364. https://doi.org/10.1073/pnas.0903410106
Smith RJ, Hopper SD, Shane MW (2011) Sand-binding roots in Haemodoraceae: global survey and morphology in a phylogenetic context. Plant Soil 348:453–470. https://doi.org/10.1007/s11104-011-0874-z
Smith SE, Read DJ (2008) Mycorrhizal Symbiosis. Academic Press and Elsevier, London
Sors TG, Ellis DR, Na GN, Lahner B, Lee S, Leustek T, Pickering IJ, Salt DE (2005) Analysis of sulfur and selenium assimilation in Astragalus plants with varying capacities to accumulate selenium. Plant J 42:785–797. https://doi.org/10.1111/j.1365-313X.2005.02413.x
Specht RL (1963) Dark Island heath (Ninety-mile plain, South Australia). VII. The effect of fertilizers on composition and growth, 1950-60. Aust J Bot 11:67–94. https://doi.org/10.1071/BT9630067
Stitt M, Lunn J, Usadel B (2010) Arabidopsis and primary photosynthetic metabolism - more than the icing on the cake. Plant J 61:1067–1091. https://doi.org/10.1111/j.1365-313X.2010.04142.x
Sulpice R, Ishihara H, Schlereth A, Cawthray GR, Encke B, Giavalisco P, Ivakov A, Arrivault S, Jost R, Krohn N, Kuo J, Laliberté E, Pearse SJ, Raven JA, Scheible WR, Teste F, Veneklaas EJ, Stitt M, Lambers H (2014) Low levels of ribosomal RNA partly account for the very high photosynthetic phosphorus-use efficiency of Proteaceae species. Plant Cell Environ 37:1276–1298. https://doi.org/10.1111/pce.12240
Tauss C, Keighery GJ, Keighery BJ, Cloran PM, Genovese SD (2019) A new look at the flora and the vegetation patterns of the Greater Brixton Street Wetlands and Yule Brook. In: Lambers H (ed) A Jewel in the Crown of a Global Biodiversity Hotspot. Kwongan Foundation and the Western Australian Naturalists’ Club Inc, Perth, pp 69–207
Teodoro GS, Lambers H, Nascimento DL, de Britto Costa P, Flores-Borges DNA, Abrahão A, Mayer JLS, Sawaya A C H F, Ladeira FSB, Abdala DB, Pérez CA, Oliveira RS (2019) Specialized roots of Velloziaceae weather quartzite rock while mobilizing phosphorus using carboxylates. Funct Ecol 33:762–773. https://doi.org/10.1111/1365-2435.13324
Tian Q, Liu N, Ma P, Zhou H, Zhai X, Chen M, Wang H, Li W, Bai W, Lambers H, Zhang W-H (2021) Processes at the soil-root interface determine the different responses of nutrient limitation and metal toxicity in forbs and grasses to nitrogen enrichment. J Ecol 109:927–938. https://doi.org/10.1111/1365-2745.13519
Tian Q, Lu P, Zhai X, Zhang R, Zheng Y, Wang H, Nie B, Bai W, Niu S, Shi P, Yang Y, Li K, Yang D, Stevens C, Lambers H, Zhang W-H (2022) An integrated belowground trait-based understanding of nitrogen driven plant diversity loss. Glob Change Biol. https://doi.org/10.1111/gcb.16147
Tjellström H, Andersson MX, Larsson KE, Sandelius AS (2008) Membrane phospholipids as a phosphate reserve: the dynamic nature of phospholipid-to-digalactosyl diacylglycerol exchange in higher plants. Plant Cell Environ 31:1388–1398. https://doi.org/10.1111/j.1365-3040.2008.01851.x
Treseder KK, Allen MF (2002) Direct nitrogen and phosphorus limitation of arbuscular mycorrhizal fungi: a model and field test. New Phytol 155:507–515. https://doi.org/10.1046/j.1469-8137.2002.00470.x
Tsujii Y, Oikawa M, Kitayama K (2017) Significance of the localization of phosphorus among tissues on a cross-section of leaf lamina of Bornean tree species for phosphorus-use efficiency. J Trop Ecol 33:237–240. https://doi.org/10.1017/s0266467417000141
Tsujii Y, Onoda Y, Kitayama K (2017) Phosphorus and nitrogen resorption from different chemical fractions in senescing leaves of tropical tree species on Mount Kinabalu. Borneo Oecologia 185:171–180. https://doi.org/10.1007/s00442-017-3938-9
Turner BL, Richardson AE (2007) Inositol phosphates in soil: amounts, forms and significance of the phosphorylated inositol stereoisomers. In: Turner BL, Richardson AE, Mullaney EJ (eds) Inositol phosphates: Linking agriculture and the environment. CAB International, p 186
Turner BL, Condron LM (2013) Pedogenesis, nutrient dynamics, and ecosystem development: the legacy of T.W. Walker and J.K. Syers. Plant Soil 367:1–10. https://doi.org/10.1007/s11104-013-1750-9
Turner BL, Hayes PE, Laliberté E (2018) A climosequence of chronosequences in southwestern Australia. Eur J Soil Sci 69:69–85. https://doi.org/10.1111/ejss.12507
Turner BL, Papházy MJ, Haygarth PM, Mckelvie ID (2002) Inositol phosphates in the environment. Philos Trans R Soc Lond B 357:449–469. https://doi.org/10.1098/rstb.2001.0837
Turner BL, Wells A, Condron LM (2014) Soil organic phosphorus transformations along a coastal dune chronosequence under New Zealand temperate rain forest. Biogeochemistry 121:595–611. https://doi.org/10.1007/s10533-014-0025-8
Twigg LE (2014) Fluoroacetate, plants, animals and a biological arms race. In: Lambers H (ed) Plant Life on the Sandplains in Southwest Australia, a Global Biodiversity Hotspot. UWA Publishing, Crawley, pp 225–240
Twigg LE, King DR (1991) The impact of fluoroacetate-bearing vegetation on native Australian fauna: A review. Oikos 61:412–430
van Blerk JJ, West AG, Altwegg R, Hoffman MT (2021) Does a trade-off between growth plasticity and resource conservatism mediate post-fire shrubland responses to rainfall seasonality? New Phytol 230:1407–1420. https://doi.org/10.1111/nph.17246
van Dam NM, Bouwmeester HJ (2016) Metabolomics in the rhizosphere: tapping into belowground chemical communication. Trends Plant Sci 21:256–265. https://doi.org/10.1016/j.tplants.2016.01.008
Van der Ent A, Baker AJM, Reeves RD, Pollard AJ, Schat H (2013) Hyperaccumulators of metal and metalloid trace elements: facts and fiction. Plant Soil 362:319–334. https://doi.org/10.1007/s11104-012-1287-3
van der Ent A, Ocenar A, Tisserand R, Sugau JB, Echevarria G, Erskine PD (2019) Herbarium X-ray fluorescence screening for nickel, cobalt and manganese hyperaccumulator plants in the flora of Sabah (Malaysia, Borneo Island). J Geochem Explor 202:49–58. https://doi.org/10.1016/j.gexplo.2019.03.013
Veneklaas EJ, Poot P (2003) Seasonal patterns in water use and leaf turnover of different plant functional types in a species-rich woodland, south-western Australia. Plant Soil 257:295–304. https://doi.org/10.1023/a:1027383920150
Verboom G, Stock W, Linder H (2002) Determinants of postfire flowering in the geophytic grass Ehrharta capensis Funct Ecol 16:705–713. https://doi.org/10.1046/j.1365-2435.2002.00673.x
Vickery B, Vickery ML (1972) Fluoride metabolism in Dichapetalum toxicarium Phytochemistry 11:1905–1909. https://doi.org/10.1016/S0031-9422(00)90151-1
Villarroel Segarra D, Wood JRI (2011) Plantago pyrophila (Plantaginaceae), a new species from the cerrados of Eastern Bolivia. Kew Bull 66:471–474. https://doi.org/10.1007/s12225-011-9298-4
Waddell HA, Simpson RJ, Ryan MH, Lambers H, Garden DL, Richardson AE (2016) Root morphology and its contribution to a large root system for phosphorus uptake by Rytidosperma species (wallaby grass). Plant Soil 1–13. https://doi.org/10.1007/s11104-016-2933-y
Walker TW, Syers JK (1976) The fate of phosphorus during pedogenesis. Geoderma 15:1–9. https://doi.org/10.1016/0016-7061(76)90066-5
Wang B, Qiu Y-L (2006) Phylogenetic distribution and evolution of mycorrhizas in land plants. Mycorrhiza 16:299–363
Wang Y, Krogstad T, Clarke JL, Hallama M, Øgaard AF, Eich-Greatorex S, Kandeler E, Clarke N (2016) Rhizosphere organic anions play a minor role in improving crop species’ ability to take up residual phosphorus (P) in agricultural soils low in P availability. Front Plant Sci 7:1664. https://doi.org/10.3389/fpls.2016.01664
Wang Y, Lysøe E, Armarego-Marriott T, Erban A, Paruch L, van Eerde A, Bock R, Liu-Clarke J (2018) Transcriptome and metabolome analyses provide insights into root and root-released organic anion responses to phosphorus deficiency in oat. J Exp Bot 69:3759–3771. https://doi.org/10.1093/jxb/ery176
Webb LJ (1954) Aluminium accumulation in the Australian-New Guinea flora. Aust J Bot 2:176–196. https://doi.org/10.1071/BT9540176
Wen Z, Li H, Shen Q, Tang X, Xiong C, Li H, Pang J, Ryan M, Lambers H, Shen J (2019) Trade-offs among root morphology, exudation and mycorrhizal symbioses for phosphorus-acquisition strategies of 16 crop species. New Phytol 223:882–895. https://doi.org/10.1111/nph.15833
Witkowski ETF, Mitchell DT (1987) Variations in soil phosphorus in the fynbos biome, South Africa. J Ecol 75:1159–1171
Yan L, Zhang X, Han Z, Lambers H, Finnegan PM (2019) Responses of foliar phosphorus fractions to soil age are diverse along a 2 Myr dune chronosequence. New Phytol 223:1621–1633. https://doi.org/10.1111/nph.15910
Yu R-P, Lambers H, Callaway RM, Wright AJ, Li L (2021) How belowground facilitation matters to enhanced biodiversity-ecosystem functioning: two or three to tango. Trends Plant Sci 26:1227–1235. https://doi.org/10.1016/j.tplants.2021.07.014
Yu R-P, Li X-X, Xiao Z-H, Lambers H, Li L (2020) Phosphorus facilitation and covariation of root traits in steppe species. New Phytol 226:1285–1298. https://doi.org/10.1111/nph.16499
Yu R-P, Zhang W, Yu Y, Yu S, Lambers H, Li L (2020) Linking shifts in species composition induced by grazing with root traits for phosphorus acquisition in a typical steppe in Inner Mongolia. Sci Tot Environ 712:136495. https://doi.org/10.1016/j.scitotenv.2020.136495
Zanin L, Venuti S, Zamboni A, Varanini Z, Tomasi N, Pinton R (2017) Transcriptional and physiological analyses of Fe deficiency response in maize reveal the presence of Strategy I components and Fe/P interactions. BMC Genomics 18:154. https://doi.org/10.1186/s12864-016-3478-4
Zemunik G, Lambers H, Turner BL, Laliberté E, Oliveira RS (2018) High abundance of non-mycorrhizal plant species in severely phosphorus-impoverished Brazilian campos rupestres. Plant Soil 424:255–271. https://doi.org/10.1007/s11104-017-3503-7
Zemunik G, Turner BL, Lambers H, Laliberté E (2015) Diversity of plant nutrient-acquisition strategies increases during long-term ecosystem development. Nat Plants 1:15050. https://doi.org/10.1038/nplants.2015.50
Zhang FS (1993) Mobilisation of iron and manganese by plant-borne and synthetic metal chelators. Plant Soil 155:111–114. https://doi.org/10.1007/bf00024996
Zhong H, Zhou J, Azmi A, Arruda AJ, Doolette AL, Smernik RJ, Lambers H (2021) Xylomelum occidentale (Proteaceae) accesses relatively mobile soil organic phosphorus without releasing carboxylates. J Ecol 109:246–259. https://doi.org/10.1111/1365-2745.13468
Zhou J, Li X-L, Peng F, Li C, Lai C, You Q, Xue X, Wu Y, Sun H, Chen Y, Zhong H, Lambers H (2021) Mobilization of soil phosphate after eight years of warming is linked to plant phosphorus-acquisition strategies in an alpine meadow on the Qinghai-Tibetan Plateau. Glob Change Biol 27:6578–6591. https://doi.org/10.1111/gcb.15914
Zhou J, Zúñiga-Feest A, Lambers H (2020) In the beginning, there was only bare regolith - then some plants arrived and changed the regolith. J Plant Ecol 13:511–516. https://doi.org/10.1093/jpe/rtaa030
Acknowledgements
We thank Xue-Meng Zhou for her comments on an earlier version of this manuscript and three anonymous Reviewers for their constructive feedback on the first submission of this manuscript. Funding was provided by Australian Research Council Grants (DP0985685, DP110101120, DP130100005, DP140100148, DP200101013, LP200100341, FT170100195 and IH140100013) and by the Deputy Vice Chancellor (Research) at the University of Western Australia.
Funding
Open Access funding enabled and organized by CAUL and its Member Institutions
Author information
Authors and Affiliations
Corresponding author
Additional information
Responsible Editor: Alexia Stokes.
Publisher’s note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/.
About this article
Cite this article
Lambers, H., de Britto Costa, P., Cawthray, G.R. et al. Strategies to acquire and use phosphorus in phosphorus-impoverished and fire-prone environments. Plant Soil 476, 133–160 (2022). https://doi.org/10.1007/s11104-022-05464-8
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11104-022-05464-8