Abstract
Aims
Struvite (MgNH4PO4.6H2O) recovered from wastewater can be used as fertilizer. The agronomic effectiveness of struvite has mostly been evaluated using ground fertilizer mixed through soil. However, fertilizers are most commonly applied in granular form in the field. In this study, we assessed the dissolution and effectiveness of different struvites when applied in granular or powdered form.
Methods
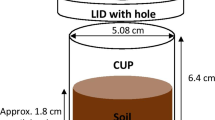
Phosphorus (P) diffusion in soil, determined using a visualization technique and chemical analyses, and P uptake by 6-week old wheat was compared for soluble fertilizer (monoammonium phosphate, MAP), a commercial struvite and three synthesized struvites with different excess MgO, in both granular and ground form.
Results
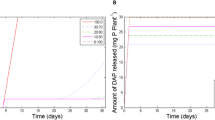
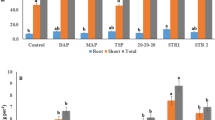
Ground struvite mixed through soil quickly dissolved and its agronomic effectiveness was similar to that of MAP. For pure granular struvite, the granule dissolution rate ranged from circa 0.03 mg d−1 in alkaline soil to 0.43 mg d−1 in acidic soil. Excess base in the struvite fertilizer reduced its dissolution rate. The P uptake by wheat followed the order MAP > > struvite ≥ control (no P), with no significant difference between the control and the struvite treatment in alkaline soil.
Conclusions
Both fertilizer characteristics (particle size, excess base) and soil pH strongly affect the dissolution rate of struvite and hence its agronomic effectiveness.






Similar content being viewed by others
References
Achat DL, Daumer ML, Sperandio M, Santellani AC, Morel C (2014) Solubility and mobility of phosphorus recycled from dairy effluents and pig manures in incubated soils with different characteristics. Nutr Cycl Agroecosyst 99:1–15
Ackerman JN, Zvomuya F, Cicek N, Flaten D (2013) Evaluation of manure-derived struvite as a phosphorus source for canola. Can J Plant Sci 93:419–424
Ahmed S, Klassen TN, Keyes S, Daly M, Jones DL, Mavrogordato M, Sinclair I, Roose T (2015) Imaging the interaction of roots and phosphate fertiliser granules using 4D X-ray tomography. Plant Soil:1–10
Alston A, Chin K (1974) Response of subterranean clover to rock phosphates as affected by particle size and depth of mixing in the soil. Anim Prod Sci 14:649–655
Antonini S, Arias MA, Eichert T, Clemens J (2012) Greenhouse evaluation and environmental impact assessment of different urine-derived struvite fertilizers as phosphorus sources for plants. Chemosphere 89:1202–1210
Babare A, Gilkes R, Sale P (1997) The effect of phosphate buffering capacity and other soil properties on North Carolina phosphate rock dissolution, availability of dissolved phosphorus and relative agronomic effectiveness. Anim Prod Sci 37:1037–1049
Barrow NJ (1985) Comparing the effectiveness of fertilizers. Fert Res 8:85–90
Bhuiyan M, Mavinic D, Beckie R (2007) A solubility and thermodynamic study of struvite. Environ Technol 28:1015–1026
Bolan N, Hedley M (1990) Dissolution of phosphate rocks in soils. 2. Effect of pH on the dissolution and plant availability of phosphate rock in soil with pH dependent charge. Fert Res 24:125–134
Bonvin C, Etter B, Udert KM, Frossard E, Nanzer S, Tamburini F, Oberson A (2015) Plant uptake of phosphorus and nitrogen recycled from synthetic source-separated urine. Ambio 44:217–227
Booker N, Priestley A, Fraser I (1999) Struvite formation in wastewater treatment plants: opportunities for nutrient recovery. Environ Technol 20:777–782
Britton A, Koch FA, Mavinic DS, Adnan A, Oldham WK, Udala B (2005) Pilot-scale struvite recovery from anaerobic digester supernatant at an enhanced biological phosphorus removal wastewater treatment plant. J Environ Eng Sci 4:265–277
Cabeza R, Steingrobe B, Römer W, Claassen N (2011) Effectiveness of recycled P products as P fertilizers, as evaluated in pot experiments. Nutr Cycl Agroecosyst 91:173–184
Capdevielle A, Sýkorová E, Biscans B, Béline F, Daumer M-L (2013) Optimization of struvite precipitation in synthetic biologically treated swine wastewater—Determination of the optimal process parameters. J Hazard Mater 244:357–369
Chimenos J, Fernandez A, Villalba G, Segarra M, Urruticoechea A, Artaza B, Espiell F (2003) Removal of ammonium and phosphates from wastewater resulting from the process of cochineal extraction using MgO-containing by-product. Water Res 37:1601–1607
Cordell D, Rosemarin A, Schröder J, Smit A (2011) Towards global phosphorus security: A systems framework for phosphorus recovery and reuse options. Chemosphere 84:747–758
Degryse F, McLaughlin MJ (2014) Phosphorus diffusion from fertilizer: visualization, chemical measurements, and modeling. Soil Sci Soc Am J 78:832–842
Grant C, Flaten D, Tomasiewicz D, Sheppard S (2001) The importance of early season phosphorus nutrition. Can J Plant Sci 81:211–224
Johnston A, Richards I (2003) Effectiveness of different precipitated phosphates as phosphorus sources for plants. Soil Use Manag 19:45–49
Kanabo I, Gilkes R (1987) The role of soil pH in the dissolution of phosphate rock fertilizers. Fert Res 12:165–173.
Kirk G, Nye P (1986) A simple model for predicting the rates of dissolution of sparingly soluble calcium phosphates in soil. I The basic model J Soil Sci 37:529–540
Kontrec J, Babić-Ivančićand V, Brečević L (2005) Formation and morphology of struvite and newberyite in aqueous solutions at 25 and 37 °C. Coll Anthropol 29:289–294
Le Corre KS, Valsami-Jones E, Hobbs P, Parsons SA (2009) Phosphorus recovery from wastewater by struvite crystallization: A review. Crit Rev Environ Sci Technol 39:433–477
Martin AE, Reeve R (1955) A rapid manometeic method for determining soil carbonate. Soil Sci 79:187–198
Matejovic I (1997) Determination of carbon and nitrogen in samples of various soils by the dry combustion. Commun Soil Sci Plant Anal 28:1499–1511
McKenzie NJ, Coughlan KJ, Cresswell HP (2002) Soil physical measurements and interpretation for land evaluation. CSIRO Publishing, Collingwood.
Nelson NO, Mikkelsen RL, Hesterberg DL (2003) Struvite precipitation in anaerobic swine lagoon liquid: effect of pH and Mg: P ratio and determination of rate constant. Bioresour Technol 89:229–236
Plaza C, Sanz R, Clemente C, Fernández JM, González R, Polo A, Colmenarejo MF (2007) Greenhouse evaluation of struvite and sludges from municipal wastewater treatment works as phosphorus sources for plants. J Agric Food Chem 55:8206–8212
Rayment GE, Higginson FR (1992) Australian laboratory handbook of soil and water chemical methods. Inkata Press, Melbourne
Robinson JS, Syers JK, Bolan NS (1992) Importance of proton supply and calcium-sink size in the dissolution of phosphate rock materials of different reactivity in soil. J Soil Sci 43:447–459
Smyth T, Sanchez P (1982) Phosphate rock dissolution and availability in Cerrado soils as affected by phosphorus sorption capacity. Soil Sci Soc Am J 46:339–345
Talboys PJ, Heppell J, Roose T, Healey J R, Jones DL, Withers PJ (2016). Struvite: A slow-release fertiliser for sustainable phosphorus management? Plant Soil 401:109–123.
Zhu YG, He YQ, Smith SE, Smith FA (2002) Buckwheat (Fagopyrum esculentum Moench) has high capacity to take up phosphorus (P) from a calcium (Ca)-bound source. Plant Soil 239:1–8
Acknowledgments
This work was supported by The Mosaic Company. We also thank Ashleigh Broadbent, Bogumila Tomczak, and Colin Rivers for their technical assistance.
Author information
Authors and Affiliations
Corresponding author
Additional information
Responsible Editor: John Hammond.
Rights and permissions
About this article
Cite this article
Degryse, F., Baird, R., da Silva, R.C. et al. Dissolution rate and agronomic effectiveness of struvite fertilizers – effect of soil pH, granulation and base excess. Plant Soil 410, 139–152 (2017). https://doi.org/10.1007/s11104-016-2990-2
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11104-016-2990-2