Abstract
Aims
Phosphorus (P) limits crop yield and P-fertilisers are frequently applied to agricultural soils. However, supplies of quality rock phosphate are diminishing. Plants have evolved mechanisms to improve P-acquisition and understanding these could improve the long-term sustainability of agriculture. Here we examined interactions between root hairs and arbuscular mycorrhizal (AM) colonisation in barley (Hordeum vulgare L.).
Methods
Barley mutants exhibiting different root hair phenotypes, wild type barley and narrowleaf plantain (Plantago lanceolata L.) were grown in the glasshouse in P-sufficient and P-deficient treatments and allowed to develop AM colonization from the natural soil community. Plants were harvested after 6 weeks growth and root hair length, AM-fungal colonisation, shoot biomass and P-accumulation measured.
Results
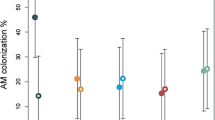
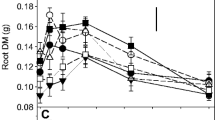
Under P-deficient conditions, root hair length and AM colonisation were negatively related suggesting that resources are allocated to root hairs rather than to AM fungi in response to P-deficiency. There was evidence that barley and narrowleaf plantain employed different strategies to increase P-acquisition under identical conditions, but root hairs were more effective.
Conclusions
This research suggests future barley breeding programmes should focus on maintaining or improving root hair phenotypes and that pursuing enhancements to AM associations under the prevalent agricultural conditions tested here would be ineffectual.




Similar content being viewed by others
Abbreviations
- NRH:
-
No root hairs
- SRH:
-
Short root hairs
- LRH:
-
Long root hairs
- WT:
-
Wild type
- PL:
-
Plantago lanceolata
- %RLC:
-
Percentage root length colonised
References
Baon JB, Smith SE, Alston AM (1993a) Mycorrhizal responses of barley cultivars differing in P-efficiency. Plant Soil 157:97–105
Baon JB, Smith SE, Alston AM (1993b) Phosphorus allocation in P-efficient and inefficient barley cultivars as affected by mycorrhizal infection. Plant Soil 156:277–280
Baon JB, Smith SE, Alston AM (1994) Growth-response and phosphorus uptake of rye with long and short root hairs–interactions with mycorrhizal infection. Plant Soil 167:247–254
Barley KP, Rovira AD (1970) The influence of root hairs on the uptake of phosphate. Commun Soil Sci Plan 1:287–292
Bates TR, Lynch JP (1996) Stimulation of root hair elongation in Arabidopsis thaliana by low phosphorus availability. Plant Cell Environ 19:529–538
Bates TR, Lynch JP (2000) The efficiency of Arabidopsis thaliana (Brassicaceae) root hairs in phosphorus acquisition. Am J Bot 87:964–970
Bates TR, Lynch JP (2001) Root hairs confer a competitive advantage under low phosphorus availability. Plant Soil 236:243–250
Baylis GTS (1970) Root hairs and phycomycetous mycorrhizas in phosphorus deficient soil. Plant Soil 33:713–716
Bhat KKS, Nye PH (1974) Diffusion of phosphate to plant roots in soil. III. Depletion around onion roots without root hairs. Plant Soil 41:383–394
Brown LK, George TS, Thompson JA, Wright G, Lyon J, Dupuy L, Hubbard SF, White PJ (2012) What are the implications of variation in root hair length on tolerence to combined abiotic stress in barley (Hordeum vulgare L.)? Ann Bot 110:319–328
Brown LK, George TS, Dupuy L, White PJ (2013) A conceptual model of root hair ideotypes for future agricultural environments–what combination of traits should be targeted to cope with limited P availability? Ann Bot. doi:10.1093/aob/mcs231
Caldwell DG, McCallum N, Shaw P, Muehlbauer GJ, Marshall DF, Waugh R (2004) A structured mutant population for forward and reverse genetics in barley (Hordeum vulgare L.). Plant J 40:143–150
Chen BD, Roos P, Borggaard OK, Zhu YG, Jakobsen I (2005) Mycorrhiza and root hairs in barley enhance acquisition of phosphorus and uranium from phosphate rock but mycorrhiza decreases root to shoot uranium transfer. New Phytol 165:591–598
Clark RB, Zeto SK (2000) Mineral acquisition by arbuscular mycorrhizal plants. J Plan Nutr 23:867–902
Dawson CJ, Hilton J (2011) Fertiliser availability in a resource-limited world: production and recycling of nitrogen and phosphorus. Food Policy 36:S14–S22
Facelli E, Smith SE, Gacelli JM, Chrisophersen HM, Smith FA (2010) Undergrould friends or enemies: model plant help to unravel direct and indirect effects of arbuscular mycorrhizal fungi on plant competition. New Phytol 185:1050–1061
Gahoonia TS, Nielsen NE (1998) Direct evidence on participation of root hairs in phosphorus (P-32) uptake from soil. Plant Soil 198:147–152
Gahoonia TS, Nielsen NE (2003) Phosphorus (P) uptake and growth of a root hairless barley mutant (bald root barley, brb) and wild type in low- and high-P soils. Plant Cell Environ 26:1759–1766
Gahoonia TS, Nielsen NE (2004) Barley genotypes with long root hairs sustain high grain yields in low-P field. Plant Soil 262:55–62
Gahoonia TS, Nielsen NE, Joshi PA, Jahoor A (2001) A root hairless barley mutant for elucidating genetic of root hairs and phosphorus uptake. Plant Soil 235:211–219
George TS, Richardson AE, Smith JB, Hadobas PA, Simpson RJ (2005) Limitations to the potential of transgenic Trifolium subterraneum L. plants that exude phytase when grown in soils with a range of organic P content. Plant Soil 278:263–274
George TS, Richardson AE, Sumei L, Gregory PJ, Daniell TD (2009) Extracellular release of a heterologous phytase from roots of transgenic plants: does manipulation of rhizosphere biochemistry impact microbial community structure? FEMS Microbiol Ecol 70:433–445
George TS, Brown LK, Newton AC, Hallett PD, Sun BH, Thomas WTB, White PJ (2011a) Impact of soil tillage on the robustness of the genetic component of variation in phosphorus (P) use efficiency in barley (Hordeum vulgare L.). Plant Soil 339:113–123
George TS, Fransson A-M, Hammond JP, White PJ (2011b) Phosphorus nutrition: rhizosphere processes, plant response and adaptations. In: Bünemann E, Oberson A, Frossard E (eds) Soil biology 26 phosphorus in action–biological processes in soil phosphorus cycling. Springer, Heidelberg, pp 245–271
Gilbert N (2009) The disappearing nutrient. Nature 461:716–718
Grace C, Stribley DP (1991) A safer procedure for routine staining of vesicular-arbuscular mycorrhizal fungi. Mycol Res 95:1160–1162
Grace EJ, Cotsaftis O, Tester M, Smith FA, Smith SE (2009) Arbuscular mycorrhizal inhibition of growth in barley cannot be attributed to extent of colonization, fungal phosphorus uptake or effects on expression of plant phosphate transporter genes. New Phytol 181:938–949
Gregory PJ, Bengough AG, Grinev D, Schmidt S, Thomas WTB, Wojciechowski T, Young IM (2009) Root phenomics of crops: opportunities and challenges. Funct Plant Biol 36:922–929
Haling RE, Simpson RJ, Delhaize E, Hocking PJ, Richardson AE (2010) Effect of lime on root growth, morphology and the rhizosheath of cereal seedlings growing in an acid soil. Plant Soil 327:199–212
Hammond JP, Broadley MR, White PJ (2004) Genetic responses to phosphorus deficiency. Ann Bot 94:323–332
Hill JO, Simpson RJ, Ryan MH, Chapman DF (2010) Root hair morphology and mycorrhizal colonisation of pasture species in response to phosphorus and nitrogen nutrition. Crop Pasture Sci 61:122–131
Hodge A (2004) The plastic plant: root responses to heterogeneous supplies of nutrients. New Phytol 162:9–24
Hodge A (2006) Plastic plants and patchy soils. J Exp Bot 57:401–411
Irving GCJ, McLaughlin MJ (1990) A rapid and simple field-test for phosphorus in Olsen and Bray No. 1 extracts of soil. Commun Soil Sci Plan 21:2245–2255
Ishida T, Kurata T, Okada K, Wada T (2008) A genetic regulatory network in the development of trichomes and root hairs. Annu Rev Plant Biol 59:365–386
Jakobsen I, Neilsen NE (1983) Vesicular-abuscular mycorrhiza in field-grown crops. I. Mycorrhizal infection in cereals and peas at various times and soil depths. New Phytol 93:401–413
Jakobsen I, Rosendahl L (1990) Carbon flow into soil and external hyphae from roots of mycorrhizal cucumber plants. New Phytol 115:77–83
Jakobsen I, Chen BD, Munkvold L, Lundsgaard T, Zhu YG (2005) Contrasting phosphate acquisition of mycorrhizal fungi with that of root hairs using the root hairless barley mutant. Plant Cell Environ 28:928–938
Johnson NC, Graham JH, Smith FA (1997) Functioning of mycorrhizal associations along the mutualism-parasitism continuum. New Phytol 135:575–586
Kormanik PP, McGraw AC (1982) Quantification of vesicular-arbuscular mycorrhizae in plant roots. In: Schenck NC (ed) Methods and principles of mycorrhizal research. The American Phytopathological Society, St. Paul, Minnesota, pp 37–45
Lewis G, Quirk JP (1967) Phosphate diffusion in soil and uptake by plants. I. Self-diffusion of phosphate in soils. Plant and Soil 26:99–118
Lynch JP (2007) Roots of the second green revolution. Aust J Bot 55:493–512
McGonigle TP, Miller MH, Evans DG, Fairchild GL, Swan JA (1990) A new method which gives an objective-measure of colonization of roots by vesicular arbuscular mycorrhizal fungi. New Phytol 115:495–501
Merryweather JW, Fitter AH (1991) A modified method for elucidating the structure of the fungal partner in a vesicular-arbuscular mycorrhiza. Mycol Res 95:1435–1437
Morgan JAW, Bending GD, White PJ (2005) Biological costs and benefits to plant-microbe interactions in the rhizosphere. J Exp Bot 56:1729–1739
Peterson RL, Farquhar ML (1996) Root hairs: specialized tubular cells extending root surfaces. Bot Rev 62:1–40
Raghothama KG (2005) Phosphorus and plant nutrition: an overview. In: Sims JT, Sharpley AN (eds) Phosphorus: agriculture and the environment. American Society of Agronomy, Madison, pp 355–378
Richardson AE, Hocking PJ, Simpson RJ, George TS (2009) Plant mechanisms to optimise access to soil phosphorus. Crop Pasture Sci 60:124–143
Schweiger PF, Robson AD, Barrow NJ (1995) Root hair length determines beneficial effect of a Glomus species on shoot growth of some pasture species. New Phytol 131:247–254
Smith SE, Read DJ (2008) Mycorrhizal symbiosis, 3rd edn. Academic, New York
Smith SE, Smith FA, Jakobsen I (2003) Mycorrhizal fungi can dominate phosphate supply to plants irrespective of growth responses. Plant Physiol 133:16–20
St John TV (1980) Root size, root hairs and mycorrhizal infection: a re-examination of Baylis’s hypothesis with tropical trees. New Phytol 84:483–487
Tiessen H (2008) Phosphorus in the global environment. In: White PJ, Hammond JP (eds) The ecophysiology of plant-phosphorus interactions. Springer, Dordrecht, pp 1–7
Vance CP, Uhde-Stone C, Allan DL (2003) Phosphorus acquisition and use: critical adaptations by plants for securing a nonrenewable resource. New Phytol 157:423–447
White PJ, Brown PH (2010) Plant nutrition for sustainable development and global health. Ann Bot 105:1073–1080
White PJ, Hammond JP (2008) Phosphorus nutrition of terrestrial plants. In: White PJ, Hammond JP (eds) The ecophysiology of plant-phosphorus interactions. Springer, Dordrecht, pp 51–81
White PJ, Hammond JP (2009) The sources of phosphorus in the waters of Great Britain. J Environ Qual 38:13–26
White PJ, Broadley MR, Greenwood DJ, Hammond JP (2005) Genetic modifications to improve phosphorus acquisition by roots. Proceedings 568. International Fertiliser Society, York
White PJ, Bengough AG, Bingham IJ, George TS, Karley AJ, Valentine TA (2009) Induced mutations affecting root architecture and mineral acquisition in barley. In: Shu QY (ed) Induced plant mutations in the genomics era. Food and Agriculture Organization of the United Nations, Rome, pp 338–340
Zygalakis KC, Kirk GJD, Jones DL, Wissuwa M, Roose T (2011) A dual porosity model of nutrient uptake by root hairs. New Phytol 192:676–688
Acknowledgments
This work was variously funded by IAEA Technical Contract 113618/RO, Scottish Government through RERAD Work package 1.7 “Profitable and sustainable agriculture” and The Royal Society of Edinburgh through a Personal Research Fellowship (TSG). Work was also supported as a MSc project by the James Hutton Institute and the University of Dundee (LKB). The authors would like to thank J. McNicol for statistical advice.
Author information
Authors and Affiliations
Corresponding author
Additional information
Responsible Editor: Matthias Wissuwa.
Rights and permissions
About this article
Cite this article
Brown, L.K., George, T.S., Barrett, G.E. et al. Interactions between root hair length and arbuscular mycorrhizal colonisation in phosphorus deficient barley (Hordeum vulgare). Plant Soil 372, 195–205 (2013). https://doi.org/10.1007/s11104-013-1718-9
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11104-013-1718-9