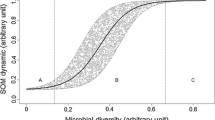
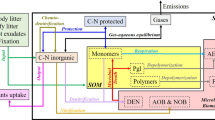
Abstract
We present a new model unifying state-of-the-art descriptions of microbial processes for denitrification, nitrification and decomposition of soil organic matter. The model is of medium complexity, filling a gap between simplistic model approaches with low predictive power and complex models, which are difficult to verify experimentally. The model Microbial Carbon and Nitrogen Turnover in soils (MiCNiT) is written in Ansi C++ and embedded into a modelling framework (MoBiLE) that provides initial conditions and accompanying ecosystem processes such as N uptake by plants, litterfall, soil water and soil temperature with established model approaches. The MiCNiT model explicitly calculates decomposition, dynamics of microbial biomass, denitrification, autotrophic and heterotrophic nitrification, applying the microbial activity concept, as well as transport of gases and solutes between anaerobic and aerobic soil fractions and through the soil profile. The model was tested against N2O and CO2 emission as well as C and N pool data from the Höglwald Forest, Germany. Due to a detailed description of the soil biochemistry and gaseous transfers, MiCNiT is capable of simulating soil air NO, N2O and N2 concentrations and the net exchange of these gases at the soil-atmosphere interface, including a possible net uptake of N2O by soils.








Similar content being viewed by others
References
Almeida JS, Reis MAM, Carrondo MJT (1995) Competition between nitrate and nitrite reduction in denitrification by Pseudomonas fluorescens. Biotechnol Bioeng 46:476–484
Almeida JS, Reis MAM, Carrondo MJT (1997) A unifying kinetic model of denitrification. J Theor Biol 186:241–249
Arah JRM, Smith KA (1989) Steady-state denitrification in aggregated soils: a mathematical model. J Soil Sci 40:139–149
Arah JRM, Vinten AJA (1995) Simplified models of anoxia and denitrification in aggregated and simple-structured soils. Eur J Soil Sci 46(4):507–517
Arneth A, Sitch S, Bondeau A, Butterbach-Bahl K, Foster P, Gedney N, de Noblet-Ducoudre N, Prentice IC, Sanderson M, Thonicke K, Wania R, Zaehle S (2010) From biota to chemistry and climate: towards a comprehensive description of trace gas exchange between the biosphere and atmosphere. Biogeosciences 7(1):121–149
Belser LW (1977) Nitrate reduction to nitrite, a possible source of nitrite for growth of nitrite-oxidizing bacteria. Appl Environ Microbiol 34:403–410
Belser LW (1984) Bicarbonate use by nitrifiers: effects of growth rate, pH, substrate concentration, and metabolic inhibitors. Appl Environ Microbiol 48:1100–1104
Belser LW, Schmidt EL (1980) Growth and oxidation-kinetics of 3 genera of ammonia oxidizing nitrifiers. FEMS Microbiol Lett 7:213–216
Betlach MR, Tiedje JM (1981) Kinetic explanation for accumulation of nitrite, nitric oxide, and nitrous oxide during bacterial denitrification. Appl Environ Microbiol 42(6):1074–1084
Blagodatsky SA, Richter O (1998) Microbial growth in soil and nitrogen turnover: a theoretical model considering the activity state of microorganisms. Soil Biol Biochem 30(13):1743–1755
Blagodatsky SA, Yevdokimov IV, Larionova AA, Richter J (1998) Microbial growth in soil and nitrogen turnover: model calibration with laboratory data. Soil Biol Biochem 30(13):1757–1764
Blagodatsky SA, Kesik M, Papen H, Butterbach-Bahl K (2006) Production of NO and N2O by the heterotrophic nitrifier Alcaligenes faecalis parafaecalis under varying conditions of oxygen saturation. Geomicrobiol J 23(3):165–176
Bollmann A, Conrad R (1998) Influence of O2 availability on NO and N2O release by nitrification and denitrification in soils. Glob Chang Biol 4(4):387–396
Bothe H, Jost G, Schloter M, Ward BB, Witzel KP (2000) Molecular analysis of ammonia oxidation and denitrification in natural environments. FEMS Microbiol Rev 24(5):673–690
Brock TD, Madigan MT (1991) Biology of microrganisms, Pretice Hall, Engelwood Cliffs, NJ
Butterbach-Bahl K, Gasche R, Willibald G, Papen H (2002) Exchange of N-gases at the Höglwald forest—a summary. Plant Soil 240:117–123
Butterbach-Bahl K, Berger U, Brüggemann N, Duyzer J (2005) Profiles of C- and N-trace gas production in N-saturated forest soils. Biogeosciences Discuss 2(4):1127–1157
Carrera J, Jubany I, Carvallo L, Chamy R, Lafuente J (2004) Kinetic models for nitrification inhibition by ammonium and nitrite in a suspended and an immobilised biomass systems. Process Biochem 39(9):1159–1165
Chapuis-Lardy L, Wrage N, Metay A, Chotte J-L, Bernoux M (2007) Soils, a sink for N2O? A review. Glob Chang Biol 13(1):1–17
Chen D, Li Y, Grace P, Mosier A (2008) N2O emissions from agricultural lands: a synthesis of simulation approaches. Plant Soil 309:169–189
Cho CM, Mills JG (1979) Kinetic formulation of the denitrification process in soil. Can J Soil Sci 59:249–257
Conrad R (2002) In: Gasche R, Papen H, Rennenberg H (eds) Microbiological and biochemical background of production and consumption of NO and N2O in soil. Kluwer Academic Publishers, Dordrecht, pp 3–33
Dassonville F, Renault P, Valles V (2004) A model describing the interactions between anaerobic microbiology and geochemistry in a soil amended with glucose and nitrate. Eur J Soil Sci 55(1):29–45
de Bruijn A, Butterbach-Bahl K (2010) Linking carbon and nitrogen mineralization with microbial responses to substrate availability—the DECONIT model. Plant Soil 328(1):271–290
de Bruijn AMG, Butterbach-Bahl K, Blagodatsky S, Grote R (2009) Model evaluation of different mechanisms driving freeze-thaw N2O emissions. Agr Ecosyst Environ 133(3–4):196–207
Del Grosso SJ, Ogle SM, Parton WJ, Breidt FJ (2010) Estimating uncertainty in N2O emissions from US cropland soils. Global Biogeochem Cy 24
Dendooven L, Splatt P, Anderson JM, Scholefield D (1994) Kinetics of the denitrification process in a soil under permanent pasture. Soil Biol Biochem 26(3):361–370
Diekkrüger B, Nörtersheuser P, Richter O (1995) Modeling pesticide dynamics of a loam site using HERBSIM and SIMULAT. Ecol Model 81(1–3):111–119
Fang C, Smith P, Smith JU, Moncrieff JB (2005) Incorporating microorganisms as decomposers into models to simulate soil organic matter decomposition. Geoderma 129:139–146
Firestone MK, Davidson EA (1989) Microbiological basis of NO and N2O production and consumption in soil. In: Andreae MO, Schimel DS (eds) Exchange of trace gases between terrestrial ecosystems and the atmosphere. Wiley, Dahlem, pp 7–21
Gignoux J, House J, Hall D, Masse D, Nacro HB, Abbadie L (2001) Design and test of a generic cohort model of soil organic matter decomposition: the SOMKO model. Glob Ecol Biogeogr 10(6):639–660
Grant RF (1995) Mathematical modelling of nitrous oxide evolution during nitrification. Soil Biol Biochem 27(9):1117–1125
Grant RF, Pattey E (1999) Mathematical modeling of nitrous oxide emissions from an agricultural field during spring thaw. Glob Biogeochem Cycles 13(2):679–694
Grant RF, Pattey E (2003) Modelling variability in N2O emissions from fertilized agricultural fields. Soil Biol Biochem 35(2):225–243
Grant RF, Shaffer M (2001) A review of the Canadian ecosystem model ecosys. In: Modelling carbon and nitrogen dynamics for soil management, CRC, Boca Raton, pp 175–264
Grant RF, Juma NG, McGill WB (1993a) Simulation of carbon and nitrogen transformations in soil: microbial biomass and metabolic products. Soil Biol Biochem 25(10):1331–1338
Grant RF, Juma NG, McGill WB (1993b) Simulation of carbon and nitrogen transformations in soil: mineralisation. Soil Biol Biochem 25(10):1317–1330
Grant RF, Nyborg M, Ladilaw JW (1993c) Evolution of nitrous oxide from soil: I. Model developement. Soil Sci 156(4):259–265
Grant RF, Nyborg M, Ladilaw JW (1993d) Evolution of nitrous oxide from soil: II. Experimental results and model testing. Soil Sci 156(4):266–277
Groffman PM (1991) Ecology of nitrification and denitrification on soil evaluated at scales relevant to atmospheric chemistry. In: EJ Rogers, WB Whitman (eds) Microbial production and consumption of greenhouse gases: methane, nitrogen oxides, and halomethanes. Washington DC: 201–221
Groffman P, Butterbach-Bahl K, Fulweiler R, Gold A, Morse J, Stander E, Tague C, Tonitto C, Vidon P (2009) Challenges to incorporating spatially and temporally explicit phenomena (hotspots and hot moments) in denitrification models. Biogeochemistry 93(1):49–77
Grote R, Lehmann E, Brummer C, Brüggemann N, Szarzynski J, Kunstmann H (2009) Modelling and observation of biosphere-atmosphere interactions in natural savannah in Burkina Faso, West Africa. Phys Chem Earth 34(4–5):251–260
Grote R, Kiese R, Grünwald T, Ourcival J-M, Granier A (2011) Modelling forest carbon balances considering tree mortality and removal. Agric For Meteorol 151(2):179–190
Heinen M (2006) Simplified denitrification models: overview and properties. Geoderma 133(3–4):444–463
Henault C, Germon JC (2000) NEMIS, a predictive model of denitrification on the field scale. Eur J Soil Sci 51(2):257–270
Hergoualc’h K, Harmand JM, Cannavo P, Skiba U, Oliver R, Henault C (2009) The utility of process-based models for simulating N2O emissions from soils: A case study based on Costa Rican coffee plantations. Soil Biol Biochem 41:2343–2355
Kesik M, Ambus P, Baritz R, Brüggemann N, Butterbach-Bahl K, Damm M, Duyzer J, Horváth L, Kiese R, Kitzler B, Leip A, Li C, Pihlatie M, Pilegaard K, Seufert G, Simpson D, Skiba U, Smiatek G, Vesala T, Zechmeister-Boltenstern S (2005) Inventories of N2O and NO emissions from European forest soils. Biogeosciences 2:353–375
Kesik M, Blagodatsky SA, Papen H, Butterbach-Bahl K (2006) Effect of pH, temperature and substrate on N2O, NO and CO2 production by Alcaligenes faecalis p. J Appl Microbiol 101:655–667
Khalil K, Renault P, Guerin N, Mary B (2005) Modelling denitrification including the dynamics of denitrifiers and their progressive ability to reduce nitrous oxide: comparison with batch experiments. Eur J Soil Sci 56(4):491–504
Koike I, Hattori A (1975) Growth yield of a denitrifying bacterium, Pseudomonas denitrificans, under aerobic and denitrifying conditions. J Gen Microbiol 88:1–10
Kreutzer K, Butterbach-Bahl K, Rennenberg H, Papen H (2009) The complete nitrogen cycle of an N-saturated spruce forest ecosystem. Plant biol (Stuttgart, Germany) 11(5):643–649
Langeveld CA, Leffelaar PA (2002) Modelling belowground processes to explain field-scale emissions of nitrous oxide. Ecol Model 149(1–2):97–112
Laughlin RJ, Rütting T, Müller C, Watson CJ, Stevens RJ (2009) Effect of acetate on soil respiration, N2O emissions and gross N transformations related to fungi and bacteria in a grassland soil. Appl Soil Ecol 42(1):25–30
Leffelaar PA (1988) Dynamics of partial anaerobiosis, denitrification, and water in a soil aggregate: simulation. Soil Sci 146(6):427–444
Leffelaar PA, Wessel WW (1988) Denitrification in a homogeneous, closed system: experiment and simulation. Soil Sci 146(5):335–349
Li C (2000) Modelling trace gas emission from agricultural ecosystems. Nutr Cycl Agroecosyst 58:259–276
Li C, Frolking S, Frolking TA (1992) A model of nitrous oxide evolution from soil driven by rainfall events: 1. model structure and sensitivity. J Geophys Res-Atmos 97(D9):9759–9776
Li C, Aber JD, Stange F, Butterbach-Bahl K, Papen H (2000) A process-oriented model of N2O and NO emissions from forest soils: 1. model development. J Geophys Res 105(D4):4369–4384
Manzoni S, Porporato A (2007) A theoretical analysis of nonlinearities and feedbacks in soil carbon and nitrogen cycles. Soil Biol Biochem 39(7):1542–1556
Manzoni S, Porporato A (2009) Soil carbon and nitrogen mineralization: theory and models across scales. Soil Biol Biochem 41(7):1355–1379
McGill WB (1996) Review and classification of ten soil organic matter (SOM) models. In: Powlson DS, Smith P, Smith JU (eds) Evaluation of soil organic matter models using existing, long-term datasets, vol I38. NATO ASI Springer Verlag, Berlin, pp 111–133
McGill WB (2007) The physiology and biochemistry of microorganisms. In: Paul EA (ed) Soil microbiology, ecology, and biochemistry. Academic, Amsterdam, pp 231–256
McKenney DJ, Drury CF, Findlay WI, Mutus B, McDonnell T, Gajda C (1994) Kinetics of denitrification by Pseudomonas fluorescens: oxygen effects. Soil Biol Biochem 26(7):901–908
Millar N, Baggs EM (2004) Chemical composition, or quality, of agroforestry residues influences N2O emissions after their addition to soil. Soil Biol Biochem 36(6):935–943
Moorhead DL, Sinsabaugh RL (2006) A theoretical model of litter decay and microbial interaction. Ecol Monogr 76(2):151–174
Müller C, Sherlock RR, Williams PH (1997) Mechanistic model for nitrous oxide emission via nitrification and denitrification. Biol Fertil Soils 24(2):231–238
Müller C, Stevens RJ, Laughlin RJ (2003) Evidence of carbon stimulated N transformations in grassland soil after slurry application. Soil Biol Biochem 35(2):285–293
Neill C, Gignoux J (2006) Soil organic matter decomposition driven by microbial growth: a simple model for a complex network of interactions. Soil Biol Biochem 38(4):803–811
Norman J, Jansson P-E, Farahbakhshazad N, Butterbach-Bahl K, Li C, Klemedtsson L (2008) Simulation of NO and N2O emissions from a spruce forest during a freeze/thaw event using an N-flux submodel from the PnET-N-DNDC model integrated to CoupModel. Ecol Model 216(1):18–30
Panikov NS (1995) Microbial growth kinetics. Chapman and Hall, Glasgow, London
Papen H, Butterbach-Bahl K (1999) A 3-year continuous record of nitrogen trace gas fluxes from untreated and limed soil of a N-saturated spruce and beech forest ecosystem in Germany—1. N2O emissions. J Geophys Res 104(D15):18487–18503
Parton WJ, Mosier A, Ojima DS, Valentine D, Schimel D, Weier KL, Kulmala AE (1996) Generalized model for N2 and N2O production from nitrification and denitrification. Glob Biogeochem Cycles 10(3):401–412
Parton WJ, Holland EA, Del Grosso SJ, Hartman MD, Martin RE, Mosier AR, Ojima DS, Schimel DS (2001) Generalized model for NOx and N2O emissions from soils. J Geophys Res 106 (D15):17,403–417,420
Paustian K (1994) Modelling soil biology and biochemical processes for sustainable agriculture research. In: Pankhurst CE, Doube BM, Gupta VVSR, Grace PR (eds) Soil biota: management in sustainable farming systems. CSIRO, Melbourne, pp 182–193
Petersen BM, Jensen LS, Hansen S, Pedersen A, Henriksen TM, Sorensen P, Trinsoutrot-Gattin I, Berntsen J (2005) CN-SIM: a model for the turnover of soil organic matter. II. Short-term carbon and nitrogen development. Soil Biol Biochem 37(2):375–393
Poth M, Focht DD (1985) 15N kinetic analysis of N2O production by Nitrosomonas europaea: an examination of nitrifier denitrification. Appl Environ Microbiol 49:1134–1141
Powell EO (1967) The growth rate of microorganisms as a function of substrate concentration. In: Powell EO (ed) Continuous cultivation of microorganisms. H.M. Stationery Office, Salisbury, pp 34–55
Renault P, Sierra J (1994) Modeling oxygen diffusion in aggregated soils: II. Anaerobiosis in topsoil layers. Soil Sci Soc Am J 58:1023–1030
Renault P, Stengel P (1994) Modeling oxygen diffusion in aggregated soils: I. Anaerobiosis inside the aggregates. Soil Sci Soc Am J 58:1017–1023
Roever M, Heinemeyer O, Kaiser EA (1998) Microbial induced nitrous oxide emissions from an arable soil during winter. Soil Biol Biochem 30(14):1859–1865
Rosenkranz P, Brüggemann N, Papen H, Xu Z, Seufert G, Butterbach-Bahl K (2006) N2O, NO and CH4 exchange, and microbial N turnover over a Mediterranean pine forest soil. Biogeosciences 3:1–13
Rosenkranz P, Dannenmann M, Brüggemann N, Papen H, Berger U, Zumbusch E, Butterbach-Bahl K (2010) Gross rates of ammonification and nitrification at a nitrogen-saturated spruce (Picea abies (L.)Karst.) stand in southern Germany. Eur J Soil Sci 61(5):745–758
Rütting T, Müller C (2007) N-15 tracing models with a Monte Carlo optimization procedure provide new insights on gross N transformations in soils. Soil Biol Biochem 39(9):2351–2361
Schimel JP, Weintraub MN (2003) The implications of exoenzyme activity on microbial carbon and nitrogen limitation in soil: a theoretical model. Soil Biol Biochem 35(4):549–563
Schurgers G, Dorsch P, Bakken L, Leffelaar P, Haugen LE (2006) Modelling soil anaerobiosis from water retention characteristics and soil respiration. Soil Biol Biochem 38(9):2637–2644
Smith KA (1980) A model of the extent of anaerobic zones in aggregated soils, and its potential application to estimates of denitrification. J Soil Sci 31:263–277
Smith OL (1982) Soil microbiology: a model of decomposition and nutrient cycling. CRC, Boca Raton
Smith P, Andren O, Brussaard L, Dangerfield M, Ekschmitt K, Lavelle P, Tate K (1998) Soil biota and global change at the ecosystem level: describing soil biota in mathematical models. Glob Chang Biol 4:773–784
Smith KA, Ball T, Conen F, Dobbie KE, Massheder J, Rey A (2003) Exchange of greenhouse gases between soil and atmosphere: interactions of soil physical factors and biological processes. Eur J Soil Sci 54:779–791
Stange CF (2007) A novel approach to combine response functions in ecological process modelling. Ecol Model 204(3–4):547–552
Stange F, Butterbach-Bahl K, Papen H, Zechmeister-Boltenstern S, Li CS, Aber J (2000) A process-oriented model of N2O and NO emissions from forest soils 2. Sensitivity analysis and validation. J Geophys Res 105(D4):4385–4398
Stark JM, Firestone MK (1996) Kinetic characteristics of ammonium-oxidizer communities in a California oak woodland-annual grassland. Soil Biol Biochem 10/11:1307–1317.
Toal ME, Yeomans C, Killham K, Meharg AA (2000) A review of rhizosphere carbon flow modelling. Plant Soil 222(1):263–281
van Cleemput O, Samater AH (1996) Nitrite in soils: accumulation and role in the formation of gaseous N compounds. Fertil Res 45(1):81–89
Van Verseveld HW, Stouthhamer AH (1978) Growth yields and the efficiency of oxidative phosphorylation during autotrophic growth of Paracoccus denitrificans on methanol and formate energy conservation during nitrate respiration. Arch Microbiol 118:21–26
Whitmore AP (1996) Describing the mineralization of carbon added to soil in crop residues using second-order kinetics. Soil Biol Biochem 28:1435–1442
WMO greenhouse gas bulletin (2010) The state of greenhouse gases in the atmosphere based on global observations through 2009. http://www.wmo.int/pages/prog/arep/gaw/ghg/documents/GHG_bull_6en.pdf Accessed 2010/12
Wolf B, Chen W, Brueggemann N, Zheng X, Pumpanen J, Butterbach-Bahl K (2011) Applicability of the soil gradient method for estimating soil atmosphere CO2, CH4 and N2O fluxes for steppe soils in Inner Mongolia. J Plant Nutr Soil Sci. doi:10.1002/jpln.201000150
Wrage N, Velthof GL, van Beusichem ML, Oenema O (2001) Role of nitrifier denitrification in the production of nitrous oxide. Soil Biol Biochem 33(12–13):1723–1732
Wu X, Brüggemann N, Gasche R, Shen Z, Wolf B, Butterbach-Bahl K (2010) Environmental controls over soil-atmosphere exchange of N2O, NO, and CO2 in a temperate Norway spruce forest. Glob Biogeochem Cycles 24(2):GB2012
Yoshinari T, Hynes R, Knowles R (1977) Acetylene inhibition of nitrous oxide reduction and measurement of denitrification and nitrogen fixation in soil. Soil Biol Biochem 9(3):177–183
Zhou Z, Zheng X, Xie B, Han S, Liu C (2010) A process-based model of N2O emission from a rice-winter wheat rotation agro-ecosystem: structure, validation and sensitivity. Adv Atmos Sci 27(1):137–150
Zumft WG (1997) Cell biology and molecular basis of denitrification. Microbiol Mol Biol Rev 61(4):553–616
Acknowledgements
We thank two anonymous reviewers for helpful comments and acknowledge the European Commission funded research project NitroEurope IP (contract 017841), and the ESF Nitrogen in Europe (NinE) program for supporting the research.
Author information
Authors and Affiliations
Corresponding author
Additional information
Responsible Editor: Ute Skiba.
Appendix
Appendix
Combined temperature and moisture reduction factor (Stange 2007):
where
- empirical function for moisture control of microbial processes in soil and fact_t is O’Neill optimum function for temperature control of biological processes (Diekkrüger et al. 1995):
Where T max —maximum temperature, T opt —optimal temperature, a—is the shape parameter of O’Neill function.
Rights and permissions
About this article
Cite this article
Blagodatsky, S., Grote, R., Kiese, R. et al. Modelling of microbial carbon and nitrogen turnover in soil with special emphasis on N-trace gases emission. Plant Soil 346, 297–330 (2011). https://doi.org/10.1007/s11104-011-0821-z
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11104-011-0821-z