Abstract
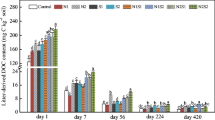
While a large number of studies have investigated the effects of macronutrients such as nitrogen (N) or phosphorus (P) on litter decomposition, recent studies suggest that micronutrients including zinc (Zn) may also limit decomposition rates. Our goal was to compare the effects of nutrient addition on decomposition of two leaf litter types from tropical dry forest trees in a short-term laboratory microcosm experiment. Single nutrients (N, P, Zn, potassium, magnesium, and nickel) were applied to leaf litter in solution at low or high concentrations (to mimic in situ availability or to alleviate nutrient limitation, respectively), and decomposition was assessed as final mass remaining and carbon dioxide mineralization. Both mass remaining and CO2 mineralization were affected by nutrient identity and concentration, and these effects varied by species. In general, P and Zn addition increased decomposition, Mg and N inhibited it, and K and Ni had no significant effects. Future studies should consider the interactions between decomposition processes, decomposer communities, and a wider range of macro- and micronutrients.

Similar content being viewed by others
References
Allison SD, Vitousek PM (2005) Responses of extracellular enzymes to simple and complex nutrient inputs. Soil Biol Biochem 37:937–944
Berg B, McClaugherty C (2008) Plant Litter, Decomposition, Humus Formation, Carbon Sequestration. Springer, Berlin
Berg B, Steffen KT, McClaugherty CA (2007) Litter decomposition rate is dependent on litter Mn concentrations. Biogeochemistry 82:29–39
Carreiro MM, Sinsabaugh RL, Repert DA, Parkhurst DF (2000) Microbial enzyme shifts explain litter decay responses to simulated nitrogen deposition. Ecology 81(9):2359–2365
Cleveland CC, Reed SC, Townsend AR (2006) Nutrient regulation of organic matter decomposition in a tropical rain forest. Ecology 87:492–503
Cleveland CC, Townsend AR, Schmidt SK (2002) Phosphorus limitation of microbial processes in moist tropical forests: evidence from short-term laboratory incubations and field studies. Ecosystems 5:680–691
Compton JE, Watrud LS, Porteous LA, DeGrood S (2004) Response of soil microbial biomass and community composition to chronic nitrogen additions at Harvard forest. For Ecol Manage 196:143–158
Cornwell WK, Cornelissen JHC, Amatangelo K, Dorrepaa E, Eviner VT, Godoy O, Hobbie SE, Hoorens B, Kurokawa H, Perez-Harguindeguy N, Quested HM, Santiago LS, Wardle DA, Wright IJ, Aerts R, Allison SD, vanBodegom P, Brovkin V, Chatain A, Callaghan TV, Dıaz S, Garnier E, Gurvich DE, Kazakou E, Klein JA, Read J, Reich PB, Soudzilovskaia NA, Vaieretti MV, Westoby M (2008) Plant species traits are the predominant control on litter decomposition rates within biomes worldwide. Ecology Letters 11:1065–1071
Dail DB, Davidson EA, Chorover J (2001) Rapid abiotic transformation of nitrate in an acid forest soil. Biogeochemistry 54:131–146
Demoling F, Nilsson LO, Baath E (2008) Bacterial and fungal response to nitrogen fertilization in three coniferous forest soils. Soil Biol Biochem 40:370–379
Duarte S, Pascoal C, Alves A, Correia A, Cassio F (2008) Copper and zinc mixtures induce shifts in microbial communities and reduce leaf litter decomposition in streams. Freshwater Biology 53:91–101
Duarte S, Pascoal C, Cassio F (2004) Effects of zinc on leaf decomposition by fungi in streams: studies in microcosms. Microbial Ecology 48:366–374
Fog K (1988) The effect of added nitrogen on the rate of decomposition of organic matter. Biological Reviews of the Cambridge Philosophical Society 63:433–462
Galicia L, Garcia-Oliva F (2004) The effects of C, N and P additions on soil microbial activity under two remnant tree species in a tropical seasonal pasture. Applied Soil Ecology 26:31–39
Gillespie TW, Grijalva A, Farris CN (2000) Diversity, composition, and structure of tropical dry forests in Central America. Plant Ecology 147:37–47
Grodziński W, Greszta J, Laskowski R, Maryański M, Rozen A (1990) Effect of the chemical composition of industrial dusts on forest floor organic matter accumulation. Water, Air, and Soil Pollution 53:169–178
Groffman PM, Fisk MC, Driscoll CT, Likens GE, Fahey TJ, Eager C, Pardo LH (2006) Calcium additions and microbial nitrogen cycle processes in a northern hardwood forest. Ecosystems 9:1289–1305
Hobbie SE (2005) Contrasting effects of substrate and fertilizer on the early stages of litter decomposition. Ecosystems 8:644–656
Hobbie SE (2008) Nitrogen effects on decomposition: a five-year experiment in eight temperate sites. Ecology 89:2633–2644
Hobbie SE, Vitousek PM (2000) Nutrient limitation of decomposition in Hawaiian forests. Ecology 81:1867–1877
Högberg P, Fan H, Quist M, Binkley D, Tamm CO (2006) Tree growth and soil acidification in response to 30 years of experimental nitrogen loading on boreal forest. Global Change Biology 12:489–499
Hughes MN, Poole RK (1989) Metals and Micro-organisms. Chapman and Hall, New York
Kaspari M, Garcia MN, Harms KE, Santana M, Wright SJ, Yavitt JB (2008) Multiple nutrients limit litter fall and decomposition in a tropical forest. Ecology Letters 11:35–43
Kaspari M, Yanoviak SP, Dudley R, Yuan M, Clay NA (2009) Sodium shortage as a constraint on the carbon cycle in an inland tropical rainforest. Proc Nat Acad Sci 106:19405–19409
Knorr M, Frey SD, Curtis PS (2005) Nitrogen additions and litter decomposition: a meta-analysis. Ecology 86:3252–3257
Lahr J, Kools SAE, vanderHout A, Faber JH (2008) Combined effects of zinc and earthworm density on soil ecosystem functioning. Soil Biol Biochem 40:334–341
Laskowski R, Maryański M, Niklińska M (1994) Effect of heavy metals and mineral nutrients on forest litter respiration rate. Environ Pollution 84:97–102
Lawrence D (2005) Regional-scale variation in litter production and seasonality in tropical dry forests of southern Mexico. Biotropica 37:561–570
LeBauer DS, Treseder KK (2008) Nitrogen limitation of net primary productivity in terrestrial ecosystems is globally distributed. Ecology 89:371–379
Lukumbuzya TK, Fyles JW, Côté B (1994) Effects of base-cation fertilization on litter decomposition in a sugar maple forest in southern Quebec. Can J For Res 24:447–452
Manzoni S, Jackson RB, Trofymow JA, Porporato A (2008) The global stoichiometry of litter nitrogen mineralization. Science 321:684–686
Marschner H (1995) Mineral nutrition of higher plants, 2nd edn. Academic, San Digeo
Martínez-Yrízar A (1995) Biomass distribution and primary productivity of tropical dry forests. In: Bullock SH, Mooney HA, Modesto E (eds) Seasonally dry tropical forests. Cambridge University Press, New York, pp 326–345
Melillo JM, Aber JD, Muratore JF (1982) Nitrogen and lignin control of hardwood leaf litter decomposition dynamics. Ecology 1982:621–626
Muneer M, Oades JM (1989) The role of Ca-organic interactions in soil aggregate stability. I. Laboratory studies with 14 C-glucose, CaCO3 and CaSO4.2H2O. Aust J Soil Res 27:389–399
Pascoal C, Cássio F, Nikolcheva L, Bärlocher F (2010) Realized fungal diversity increases functional stability of leaf litter decomposition under zinc stress. Microb Ecol 59:84–93
Powers JS, Becknell JM, Irving J, Perez-Aviles D (2009) Diversity and structure of regenerating tropical dry forests in Costa Rica: geographic patterns and environmental drivers. For Ecol Manage 258:959–970
Promputtha I, Lumyong S, Dhanasekaran V, McKenzie EHC, Hyde KD, Jeewon R (2007) A phylogenetic evaluation of whether endophytes become saprotrophs at host senescence. Microb Ecol 53:579–590
Reich PB, Oleksyn J (2004) Global patterns of plant leaf N and P in relation to temperature and latitude. Proc Nat Acad Sci 101:11001–11006
Ruhling A, Tyler G (1973) Heavy metal pollution and decomposition of spruce needle litter. Oikos 24:402–416
Sinsabaugh RL (1994) Enzymic analysis of microbial pattern and process. Biol Fert Soils 17:69–74
Sinsabaugh RL, Moorhead DL (1994) Resource allocation to extracellular enzyme production: a model for nitrogen and phosphorus control of litter decomposition. Soil Biol Biochem 26:1305–1311
Sinsabaugh RL, Gallo ME, Lauber C, Waldrop MP, Zak DR (2005) Extracellular enzyme activities and soil organic matter dynamics for northern hardwood forests receiving simulated nitrogen deposition. Biogeochemistry 75:201–215
Strickland MS, Lauber C, Fierer N, Bradford MA (2009) Testing the functional significance of microbial community composition. Ecology 90:441–451
Strojan CL (1978) Forest leaf litter decomposition in the vicinity of a zinc smelter. Oecologia 32:203–212
Swift MJ, Heal OW, Anderson JM (1979) Decomposition in terrestrial ecosystems. Studies in Ecology, vol 5. University of California Press, Berkeley
Townsend AR, Cleveland CC, Houlton BZ, Alden CB, White JWC (2011) Multi-element regulation of the tropical forest carbon cycle. Front Ecol Environ 9:9–17
Treseder KK (2008) Nitrogen additions and microbial biomass: a meta-analysis of ecosystem studies. Ecol Lett 11:1111–1120
Tyler G (1974) Heavy metal pollution and soil enzymatic activity. Plant Soil 41:303–311
Tyler G (2005) Changes in the concentrations of major, minor and rare-earth elements during leaf senescence and decomposition in a Fagus sylvatica forest. For Ecol Manage 206:167–177
Vadeboncoeur MA (2010) Meta-analysis of fertilization experiments indicates multiple limiting nutrients in northeastern deciduous forests. Can J For Res 40:1766–1780
VanSoest PJ (1967) Development of a comprehensive system of feed analysis and its application to forages. J Animal Sci 26:119–128
VirzoDeSanto A, DeMarco A, Fierro A, Berg B, Rutigliano FA (2009) Factors regulating litter mass loss and lignin degradation in late decomposition stages. Plant Soil 318:217–228
Vitousek PM (1984) Litter fall, nutrient cycling, and nutrient limitation in tropical forests. Ecology 65:285–298
Vitousek PM (1998) Foliar and litter nutrients, nutrient resorption, and decomposition in Hawaiian Metrosideros polymorpha. Ecosystems 1:401–407
Vitousek PM, Farrington H (1997) Nutrient limitation and soil development: experimental test of a biogeochemical theory. Biogeochemistry 37:63–75
Wackett LP, Dodge AG, Ellis LBM (2004) Microbial genetics and the periodic table. Appl Environ Microbiol 70:647–655
Wackett LP, Orme-Johonson WH, Walsh CT (1989) Transition metal enzymes in bacterial metabolism. In: Beveridge TJ, Doyle RJ (eds) Metal Ions and Bacteria. Wiley, New York, pp 165–206
Wallenstein MD, McNulty S, Fernandez IJ, Boggs J, Schlesinger WH (2006) Nitrogen fertilization decreases forest soil fungal and bacterial biomass in three long-term experiments. For Ecol Manage 222:459–468
Acknowledgements
Funding for this study was provided through a NASA New Investigator Award (NS000107) to J. Powers. We thank Peter Tiffin, Carol Adair, David Manning, and two anonymous reviewers for reviews of previous drafts of this manuscript and Jennifer King for allowing us to use her GC.
Author information
Authors and Affiliations
Corresponding author
Additional information
Responsible Editor: Tim Moore.
Rights and permissions
About this article
Cite this article
Powers, J.S., Salute, S. Macro- and micronutrient effects on decomposition of leaf litter from two tropical tree species: inferences from a short-term laboratory incubation. Plant Soil 346, 245–257 (2011). https://doi.org/10.1007/s11104-011-0815-x
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11104-011-0815-x