Abstract
Key message
Calmodulin-like-proteins (CML) belong to a family of calcium-sensing proteins that are unique for plants and involved in many different developmental and stress-related reactions. In defense against herbivory, some pathogens and drought, CML37 acts as a positive and CML42 as a negative regulator, respectively. We provide evidence that both CMLs act antagonistically in the regulation of induced defense responses. A double knock-out line, cml37 x cml42, thus shows wild-type phenotypes upon all kind of stresses we used.
Abstract
A transient increase in the cytosolic calcium concentration is one of the first reactions that can be measured in plant cells upon abiotic as well as biotic stress treatments. These calcium signals are sensed by calcium binding proteins such as calmodulin-like proteins (CMLs), which transduce the sensed information into appropriate stress responses by interacting with downstream target proteins. In previous studies, CML37 has been shown to positively regulate the plants’ defense against both the insect herbivore Spodoptera littoralis and the response to drought stress. In contrast, CML42 is known to negatively regulate those two stress responses. Here, we provide evidence that these two CMLs act antagonistically in the regulation of induced responses directed against drought and herbivory stress as well as in the defense against the necrotrophic pathogen Alternaria brassicicola. Both CMLs shape the plant reactions by altering the phytohormone signaling. Consequently, the phytohormone-regulated production of defensive compounds like glucosinolates is also antagonistically mediated by both CMLs. The finding that CML37 and CML42 have antagonistic roles in diverse stress-related responses suggests that these calcium sensor proteins represent important tools for the plant to balance and fine-tune the signaling and downstream reactions upon environmental stress.
Similar content being viewed by others
Introduction
Plants are faced with a multiplicity of environmental changes at the same time. In order to adapt to their changing environment, each of these stimuli needs to be perceived and translated into an appropriate response via complex signaling networks. Calcium (Ca2+) as a second messenger plays a central role in these signaling networks. In response to various abiotic and biotic stimuli, changes in the cytosolic Ca2+ concentration are reported, e.g. after drought and salinity stress (Knight et al. 1997), extreme temperature fluctuations (Knight et al. 1991; Plieth et al. 1999), light (Shacklock et al. 1992), mechanical stimulation (Knight et al. 1991) as well as after interaction with symbionts (Ehrhardt et al. 1996; Vadassery et al. 2009), pathogens-derived elicitors (Knight et al. 1991) or herbivores (Maffei et al. 2004). Each of those stimuli induces a Ca2+ oscillation in the cell, the so called Ca2+ signature, that can differ in location, duration, amplitude and frequency, reflecting the strength and the type of the stimulus (McAinsh et al. 1997). Together with other cellular messengers, such as reactive oxygen species, pH changes and membrane potential changes, these complex spatio-temporal Ca2+ signatures form a code, transferring specific information about the environment into the plant cell (McAinsh et al. 1997; Plieth 2016). In order to translate this code into the respective response of the plant, the cellular changes need to be sensed.
Calcium (Ca2+) oscillations are sensed by Ca2+ binding proteins. These proteins possess Ca2+ binding motifs, consisting out of two helices, the E- and the F-helix, connected via a loop-structure and thus called EF-hand (Kretsinger and Nockolds 1973). An EF-hand binds a single Ca2+ ion (Kretsinger and Nockolds 1973), leading to a conformational change of the Ca2+ sensor protein that allows subsequently the interaction with a certain target. Some sensors, so-called sensor responders, have additionally enzymatic domains, like the Ca2+-dependent protein kinases (CDPKs) in plants that are activated upon binding of Ca2+. However, most Ca2+ sensing proteins just possess EF-hands as functional domains and thus are dependent on an interacting protein to transduce the sensed signal into a response. In plants three major groups of these sensor relays are distinguished: calmodulins (CaMs), calmodulin-like proteins (CMLs) and calcineurin B-like proteins (CBLs) (Sanders et al. 2002).
Amongst them, the family of CMLs is of special interest for decoding calcium signatures upon stress in plants, since they are unique to the plant kingdom (Bender and Snedden 2013). Further, in comparison to the related CaMs, CMLs display a much more distinctive expression pattern upon various stress treatments in Arabidopsis thaliana, suggesting that they might play a role in decoding calcium signals upon stress in plants (McCormack et al. 2005). For a few members of the CML family, there is also evidence that they mediate responses to various stresses in A. thaliana, although for most CMLs a functional characterization is still missing. Recently it was shown that CML41 reduces bacterial infection of Pseudomonas syringae by regulating the closure of plasmodesmata (Xu et al. 2017). Also CML8, CML9 and CML24 are known to positively regulate the immune response to P. syringae (Leba et al. 2012; Ma et al. 2008; Zhu et al. 2017). Besides, CML9 and CML24 have been shown to mediate the salt stress response in A. thaliana (Delk et al. 2005; Magnan et al. 2008) and thus seem to play a role in abiotic as well as biotic stress regulation. Similarly, CML37 and CML42 are known to be important in regulating both, abiotic and biotic stress responses. They mediate the defense against the lepidopteran herbivore Spodoptera littoralis and the drought stress reaction of A. thaliana (Scholz et al. 2014, 2015; Vadassery et al. 2012). In both stress treatments it has been shown that CML37 and CML42 act via altering the phytohormone signaling in the plant. In response to S. littoralis feeding, loss-of-function mutants of CML37 accumulated less jasmonates, leading to a higher susceptibility of the plant to the herbivore (Scholz et al. 2014). In contrast, knock out mutants of CML42 showed an upregulation of jasmonate-dependent defense responses, but a wild type-like jasmonate elevation, suggesting hypersensitivity to jasmonates and causing a higher resistance to the herbivore (Vadassery et al. 2012). Similarly, cml42 accumulated higher levels of abscisic acid (ABA) upon drought conditions and thus was more resistant to drought, whereas cml37 displayed no ABA response at all and was highly more susceptible (Scholz et al. 2015; Vadassery et al. 2012).
Since CML42 turned out to be a negative regulator of both herbivore and drought stress tolerance and CML37 a positive one, it was hypothesized that they might be antagonists in regulating these stress responses (Scholz et al. 2014, 2015). To examine the interplay of CML42 and CML37, we constructed a double knock out mutant line and analyzed the response of this line upon drought and S. littoralis feeding in order to directly connect this investigation with the former studies. We show that effects of cml37 abolish the effects of cml42 in the double knock out mutant line and vice versa, verifying that CML37 and CML42 act antagonistically in regulating both stress responses. Further, we included infection with the necrotrophic fungal pathogen A. brassicicola in order to extend the study with a different type of stress. We demonstrate that CML37 and CML42 also regulate the defense against A. brassicicola antagonistically, suggesting a general antagonistic role of CML37 and CML42 in the regulation of the jasmonate-dependent defense responses. By studying a double knock out mutant of both CMLs we are now able to refine the roles of CML37 and CML42 in balancing different stress responses.
Materials and methods
Plant materials
Arabidopsis plants were grown under short day conditions at the MPI CE Jena in round pots with 10 cm diameter as described in Heyer et al. (2018). The double knock out mutant line cml37 × cml42 was obtained by crossing cml37-1 (SALK_011488C) (Scholz et al. 2014) and cml42 (SALK_041400C) (Dobney et al. 2009; Vadassery et al. 2012). Plants were selected by genotyping and selection was proven by RT-PCR. Experiments were performed with the F4 and F5 progeny of the crossed plants. Arabidopsis thaliana ecotype Col-0 was used as control.
Fungus treatments were performed at the FSU Jena and plants were grown on plates as described in Johnson et al. (2013). After 21 days plants were transferred to soil and further cultivated as described in Heyer et al. (2018).
Insect rearing and oral secretion (OS) collection
Spodoptera littoralis eggs were obtained from Syngenta Crop Protection AG (Stein, Switzerland). Larvae were reared on an artificial diet based on ground beans modified after Bergomaz and Boppré (1986) at 23–25 °C and with 14 h photoperiod. Modifications of the diet composition are described in Heyer et al. (2018). For collection of OS, fourth instar larvae were starved overnight and were allowed to feed on the respective plant genotype for one day. OS was collected on ice and centrifuged at 13,000 rpm for 5 min after collection (Vadassery et al. 2012). It was stored at − 80 °C until use.
Fungal growth
Alternaria brassicicola (FSU-218) was obtained from Jena Microbial Resource Center (Jena, Germany). Fungus was grown according to Heyer et al. (2018).
Plant treatments
All herbivore-related experiments were done with 5–6 week old plants. One week feeding assays were performed with first instar larvae as described in Vadassery et al. (2012) (see insect biomass assay). For short term feeding assays fourth instar larvae were used. To ensure sufficient feeding, larvae were starved 12 h prior the assays and three larvae were allowed to feed on one plant (Scholz et al. 2014; Vadassery et al. 2012). OS treatments were done as described in Vadassery et al. (2012).
Four week old plants were used for drought stress assays. Drought was applied for 1 or 2 weeks. If applied for 2 weeks, plants were re-watered after first week of drought stress as described in Scholz et al. (2015). Control and mutant plants were kept randomly distributed on the same tray to minimize experimental variation.
Plant material collected for metabolite quantification or RT-PCR was immediately frozen in liquid nitrogen and stored at − 80 °C until extraction.
For pathogen treatments detached, fully expanded leaves of 5–6 week old plants were used. Alternaria treatments were carried out as described in Heyer et al. (2018).
Genotyping
Single leaves of 3 week old plants were cut and immediately frozen in liquid nitrogen. Plant material was ground using 2010 Geno/Grinder® (SPEX®SamplePrep, Metuchen USA). To avoid defrosting of the samples, they were stored in precooled aluminum racks throughout grinding process. DNA was extracted after a modified protocol of Konieczny and Ausubel (1993). Modifications are described in Heyer et al. (2018). PCR was performed using native Taq DNA polymerase and 10 mM dNTP Mix from Invitrogen™ by Thermo Fisher Scientific (Carlsbad, USA). PCR mix was prepared according to manufacturers’ protocol. The total reaction volume was scaled down to 10 µl, including 1.5 µl of template. Primers published in Scholz et al. (2014) and Vadassery et al. (2012) were used.
Semi-quantitative reverse transcription (RT)-PCR
Treated leaves were sampled and ground as described above. RNA was isolated using TRIzol® Reagent (Invitrogen™ by Thermo Fisher Scientific, Carlsbad, USA). Extraction was performed according to the manufacturers’ protocol with modifications as described in Heyer et al. (2018). To avoid DNA contamination, extracted RNA was treated with TURBO DNase (TURBO DNA-free™ Kit, Invitrogen™ by Thermo Fisher Scientific, Vilnius, Lithuania). PCR was done as described above. ACTIN2 was used as housekeeping gene. Primers for CML37 as published in Scholz et al. (2014) and CML42 and ACTIN2 as described in Vadassery et al. (2012) were used.
Phytohormone quantification
Phytohormones were extracted from treated leaves using the protocol described in Jimenez-Aleman et al. (2015) with slight modifications. Approximately 250 mg of ground leaf material was extracted using 1.5 ml methanol containing 60 ng D6-ABA (Santa Cruz Biotechnology, Santa Cruz, USA), 60 ng of D6-jasmonic acid (HPC Standards GmbH, Cunnersdorf, Germany), 60 ng D4-salicylic acid (Sigma-Aldrich, St. Louis, USA) and 12 ng of jasmonoyl-13C6-isoleucine [synthesized as described in Kramell et al. (1988)] as internal standard. Phytohormone analysis was performed according to the protocol of Vadassery et al. (2012). Protocol was modified as follows. For herbivore treated samples chromatography was performed on an Agilent 1260 HPLC system (Agilent Technologies, Santa Clara, USA), for drought stress samples chromatographic separation was done on an Agilent 1200 HPLC (Agilent Technologies, Santa Clara, USA) using a Zorbax Eclipse XDB-C18 column (50 × 4.6 mm, 1.8 µm, Agilent Technologies, Santa Clara, USA) in both cases. Water containing 0.05% formic acid and acetonitrile were employed as mobile phases A and B, respectively. The elution profile for herbivore treated samples was: 0–0.5 min, 5% B; 0.5–9.5 min, 5–42% B; 9.5–9.51 min, 42–100% B; 9.51–12 min 100% B and 12.1–15 min, 5% B. In case of drought stress treated samples elution profile was: 0–0.5 min, 10% B; 0.5–4.0 min, 10–90% B; 4.0–4.02 min, 90–100% B; 4.02–4.5 min, 100% B and 4.51–7.0, min 10% B. Flow rate was kept at 1.1 ml/min and column temperature was maintained at 25 °C. Mass spectrometry of herbivore treated samples was performed on an API 5000 tandem mass spectrometer (Applied Biosystems™, Darmstadt, Germany) and on an API 3200 tandem mass spectrometer (Applied Biosystems™, Darmstadt, Germany) in case of drought stressed samples. Both spectrometers were equipped with a Turbo spray ion source operated in negative ionization mode. The ion spray voltage was maintained at − 4500 eV. The turbo gas temperature was set at 700 °C. Nebulizing gas was set at 60 psi, curtain gas at 25 psi, heating gas at 60 psi, and collision gas at 7 psi. The following analyte parent ion → product ion fragmentations were used for multiple reaction monitoring (MRM): mass-to-charge ratio (m/z) 263.0 → 153.2 [collision energy (CE) − 22 V; declustering potential (DP) − 35 V] for ABA; m/z 269.0 → 159.2 (CE − 22 V; DP − 35 V) for D6-ABA; m/z 209.1 → 59.0 (CE − 24 V; DP − 35 V) for jasmonic acid (JA); m/z 215.1 → 59.0 (CE − 24 V; DP − 35 V) for D6-JA; m/z 136.9 → 93.0 (CE − 22 V; DP − 35 V) for salicylic acid (SA); m/z 140.9 → 97.0 (CE − 22 V; DP − 35 V) for D4-SA; m/z 290.9 → 165.1 (CE − 24 V; DP − 45 V) for cis-12-oxophytodienoic acid (OPDA), m/z 322.2 → 130.1 (CE − 30 V; DP − 50 V) for jasmonoyl isoleucine (JA-Ile); m/z 328.2 → 136.1 (CE − 30 V; DP − 50 V) for JA-13C6-Ile. Both Q1 and Q3 quadrupoles were maintained at unit resolution. Analyst 1.5 software (Applied Biosystems™, Darmstadt Germany) was used for data acquisition and processing. Linearity in ionization efficiencies was verified by analyzing dilution series of standard mixtures. Phytohormones were quantified relative to the signal of their corresponding internal standard. For quantification of OPDA the internal D6-JA standard was used applying experimental-determined response factors of 0.5 respectively. The response factor was determined by analyzing a mixture of cis-OPDA [kindly provided by W. Boland, MPI for Chemical Ecology, Jena, Germany; synthesized as described in Shabab et al. (2014)] and D6-JA all at the same concentration. For JA-Ile quantification after herbivory only the peak of the endogenous bioactive ( +)-7-iso-jasmonoyl-L-isoleucine (Fonseca et al. 2009) was used.
Quantification of glucosinolates
Whole Arabidopsis rosettes where collected and freeze dried, to avoid fast degradation of glucosinolates. Freeze-dried samples were ground to a fine powder in uncooled racks in 2010 Geno/Grinder® (SPEX®SamplePrep, Metuchen USA). Extraction was performed according to Burow et al. (2006) with some modifications. For each sample, approximately 25 mg per sample were extracted in 1 ml 80% methanol containing 50 µM 4-hydroxybenzylglucosinolate [isolated from Sinapis alba seeds according to Thies (1988)] as internal standard. Samples were mixed for 10 min at room temperature and pelleted by centrifugation. 800 µl of the supernatant was transferred to columns containing 28 mg DEAE Sephadex A25 (Sigma-Aldrich, St. Louis, USA) each. Columns were prewashed with 800 µl water and 500 µl 80% methanol. After loading the samples, columns were washed with 500 µl 80% methanol and twice with 1 ml water. Afterwards they were rinsed with 500 µl 0.02 M MES buffer (pH 5.2) and 30 µl of sulfatase (from Helix pomatia, Sigma Aldrich, St. Louis, USA) was applied to the columns. Sulfatase was prepared according to Graser et al. (2000). Columns were incubated for desulfation at room temperature overnight and desulfoglucosinolates were eluted with 500 µl water. Desulfoglucosinolates were analyzed using HPLC/UV and quantified as described in Vadassery et al. (2012).
Chlorophyll fluorescence measurements
Chlorophyll fluorescence parameters was measured in a FluorCam FC 800-C (Psi, Drasov, Czech Republic) as described in Heyer et al. (2018).
Statistical analysis
Statistics were performed using R 3.5.1 (R Development Core Team 2018) and SigmaPlot 11.0 (Systat Software 2008). Differences in larval weight of S. littoralis were tested by Wilcoxon-test. Differences in phytohormone concentration between two genotypes were determined with Wilcoxon-test or t-test depending on the homogeneity and normal distribution of the data. In order to test whether the glucosinolate content, chlorophyll fluorescence and ABA content differed between different genotypes with different treatments, two-way ANOVAs was applied, followed by Tukey tests as post-hoc test if necessary In case of variance inhomogeneity, generalized least square method [gls from the nlme library (Pinheiro et al. 2018)] with varIdent function was applied instead of a two-way ANOVA. Whether the different variance of genotype, treatment, or the combination of both factors should be incorporated into the model was determined by comparing different variance structure models, selecting for the model with the lowest Akaike Information Criteria (AIC) (Zuur et al. 2009). The influence of the different genotypes, treatments and the interaction of both was determined by likelihood ratio tests following the backward selection protocol of Zuur et al. (2009). To test for differences among the groups factor level reduction was used (Crawley 2013). In order to obtain normal distribution of residuals, data were transformed before applying the statistic test, when it was necessary. The respective statistical test and transformation method is mentioned in the respective tables. Total number of replicates (n) is indicated in the figure legend. All experiments shown are repeated at least two times independently.
Results
Construction of the cml37 × cml42 double knock out line
To investigate the possible antagonism of CML37 and CML42, a loss-of-function mutant of both proteins was generated by crossing the T-DNA insertion lines cml37-1 (Scholz et al. 2014) and cml42 (Dobney et al. 2009; Vadassery et al. 2012). The homozygosity of the crossed line was confirmed by genotyping (Fig. 1A). For both genes the product for the intact gene was not detectable, whereas the product including the left border of the T-DNA was detectable in both cases (Fig. 1A), showing that cml37 × cml42 is homozygous.
Genetic characterization of cml37 × cml42. A Results of Genotyping cml37 × cml42. Different numbers indicate different Primer-sets used: (1) CML42 RP + CML42 LP (expected product size: 1120 bp) to verify the presence of the wild type CML42 (2) CML37 RP + CML37 LP (expected product size: 1158 bp) to verify the presence of the wild type CML37 (3) CML42 RP + LBb1.3 (expected product size: 456–756 bp) to verify the presence of the T-DNA insertion in CML42 (4) CML37 RP + LBb1.3 (expected product size: 599–899 bp) to verify the presence of the T-DNA insertion in CML37 (B) RT-PCR to confirm the absence of the CML37 and CML42 transcripts in cml37 × cml42. Plants were wounded with a pattern wheel and treated with oral secretion (W + OS) for 1 h or were used untreated. Water was used as negative control. Expected product size is indicated to the right of the gel pictures. ACTIN2 expression was used as quantitative control
Further, the absence of CML37 and CML42 transcript was confirmed by RT-PCR. Since the constitutive expression level of CML37 in adult leaves is comparatively low (McCormack et al. 2005), plants were wounded and treated with S. littoralis OS to stimulate the expression of the CMLs. Whereas both CMLs are expressed in untreated and treated wild type plants, no product was detected in case of the double knock out mutant (Fig. 1B), confirming that it is a loss of function of both CMLs.
S. littoralis performance is not affected in cml37 × cml42
To test the hypothesis of the possible antagonism of CML37 and CML42 in the herbivore defense, the cml37 × cml42 mutant line was used for insect performance assays. First instar S. littoralis larvae were allowed to feed on wild type and mutant plants for 1 week. The insect performance was evaluated by the gain of weight. Results are presented in Fig. 2. After 1 week, S. littoralis larvae gained as much weight on cml37 × cml42 as on wild type plants, suggesting the positive effect of cml37 and the negative effect of cml42 on the larval weight gain (Scholz et al. 2014; Vadassery et al. 2012) compensate each other in the double knock out mutant. Thus, both CMLs seem to be antagonistic in regulating the herbivore defense of A. thaliana.
Feeding performance of S. littoralis larvae on cml37 × cml42. Larval weight gain in mg ± Standard error (SE) after 1 week of feeding on either cml37 × cml42 or wild type (WT) plants. First instar S. littoralis larvae were pre-weighed to reduce experimental variation. Three larvae were placed on each plant. After 1 week of feeding, larval weight was determined. Experiment was repeated five times independently [n = 138 (Larvae on WT), n = 140 (Larvae on cml37 × cml42)]. Larval weights of both genotypes were compared; n.s. means not significant (p = 0.9589)
cml37 × cml42 displays a wild type-like phytohormone response after herbivory
In former studies, the higher susceptibility of cml37 to S. littoralis was shown to be caused by a lower level of the jasmonate-precursor OPDA and the active jasmonate JA-Ile (Scholz et al. 2014). However, in cml42, there was no difference in phytohormone concentration between the wild type and the mutant (Vadassery et al. 2012). Thus, we investigated the phytohormone levels after S. littoralis feeding on cml37 × cml42. Similar to both single mutant lines, the levels of SA and JA did not change in the double knock out mutant line compared to the wild type (Fig. 3A, C, Table 1). However, in contrast to the single mutant lines, cml37 × cml42 plants also displayed wild type-like OPDA and JA-Ile concentrations in non-treated controls and after S. littoralis feeding (Fig. 3B, D, Table 1). Even though CML42 was shown to have no effect on phytohormone levels itself (Vadassery et al. 2012), cml42 is able to rescue the negative effect of cml37 on the OPDA and JA-Ile levels in cml37 × cml42 after insect feeding.
Phytohormone contents of cml37 × cml42 after feeding of S. littoralis larvae. Contents of A SA, B OPDA, C JA, and D JA-Ile in ng/g fresh weight (FW) ± SE in cml37 x cml42 and WT plants after S. littoralis feeding. Larvae were allowed to feed for 3 h, 24 h and 48 h on the plants. Phytohormones were analyzed from local fed leaves. Untreated plants were used as controls (0 h). Experiment was repeated four times independently (n ≥ 10). Phytohormone contents of both genotypes within the time point were compared; n.s. means not significant. The respective p-values are given in Table 1. Legend for color code see (A)
Induction of secondary metabolites is altered in cml37 × cml42
Vadassery et al. (2012) published that the higher resistance of cml42 to insect feeding is, inter alia, due to a higher constitutive level of defensive compounds, the glucosinolates. On the other hand, loss-of-function mutants of CML37 displayed constitutive and induced glucosinolate levels that were comparable to those of wild type plants (Scholz et al. 2014). In order to investigate if cml37 is able to rescue the glucosinolate phenotype of cml42, we measured the glucosinolate content of cml37 × cml42 before and after S. littoralis feeding. There were no differences in the total glucosinolate levels of untreated 5–6 week-old cml37 × cml42 and wild type plants (Fig. 4A, Table 2). Since the higher total constitutive glucosinolate content in cml42 was due to an increased level of aliphatic glucosinolates (Vadassery et al. 2012), we further distinguished into aliphatic and indole glucosinolates. Untreated 5 and 6 week-old cml37 × cml42 plants displayed a wild type-like constitutive level of aliphatic and indole glucosinolates, showing that cml37 counteracts the effect on glucosinolates of cml42 in the double knock out mutant line (Fig. 4B, C, Table 2). Further, no differences in the induced total, aliphatic or indole glucosinolate content were measured after 1 day feeding between cml37 × cml42 and wild type plants (Fig. 4, Table 2). However, after 1 week of feeding, the induction of glucosinolates in cml37 × cml42 was significantly lower than in wild type plants (Fig. 4A, Table 2). This seems to be due to both the lower level in aliphatic as well as in indole glucosinolates (Fig. 4B, C). Although the interaction of genotype and treatment was not significant in case of the aliphatic glucosinolates there is a clear tendency (Table 2). This result seems to be a secondary effect only detected in the double knock out mutant, since neither cml37 nor cml42 were impaired in the induction of glucosinolates (Scholz et al. 2014; Vadassery et al. 2012). However, the lower induction of glucosinolates after 7d does not cause better larval performance after 1 week (Fig. 2). Thus, it is unclear if this late reduction of the glucosinolates content in cml37 × cml42 is of functional relevance for the insect defense.
Glucosinolate content of cml37 × cml42 after feeding of S. littoralis larvae. A Total glucosinolate, B aliphatic glucosinolate and C indole glucosinolate contents of WT and cml37 × cml42 plants in µmol/g dry weight (DW) ± SE after 1 days (5 week old plants) and 7 days (6 week old plants) of S. littoralis feeding. Untreated plants were used as controls. Glucosinolates were extracted from whole Arabidopsis rosettes. Experiment was repeated at least four times independently (n ≥ 14). Differences between glucosinolate contents of controls, treated samples and genotypes were determined within one time point by two-way ANOVA (A, B) and generalized least square method (C). Differences between the groups are indicated by different letters above the bars (p-value ≤ 0.05). Details about all the statistic tests including the respective statistical values and especially for 7 days aliphatic glucosinolates, are listed in Table 2. Legend for color code see (A)
cml37 and cml42 display a different susceptibility to A. brassicicola
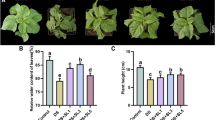
Besides mediating the defense against herbivores, jasmonates are also important signaling components in the defense against necrotrophic pathogens (Glazebrook 2005; Thomma et al. 1998). Since both CML37 and CML42 are regulating the defense against the herbivore S. littoralis by affecting the jasmonate pathway (Scholz et al. 2014; Vadassery et al. 2012), we examined whether they are influencing the resistance to the necrotrophic fungus A. brassicicola as well. In line with the results from the herbivore assays, both cml mutants displayed an antagonistic phenotype. Whereas cml37 was much more susceptible to the fungus treatment, cml42 seemed to be more resistant than the wild type (Fig. 5A). After 3 days of treatment, necrotic lesions were slightly larger on cml37 than on wild type leaves (Fig. 5A). After 4 days, the necrotic area was even covering nearly the whole cml37 leaf, whereas just a part of the wild type leaf was necrotic (Fig. 5A). On the other hand, lesions on cml42 leaves were smaller than on wild type plants at both time points (Fig. 5A). Interestingly cml37 × cml42 displayed an intermediate lesion forming phenotype that was comparable to wild type plants (Fig. 5A), suggesting again that the negative effect of cml37 and the positive effect of cml42 are neutralizing each other in the double mutant.
Different susceptibility of cml37, cml42 and cml37 × cml42 to A. brassicicola. A Necrotic lesion phenotype and B chlorophyll fluorescence (QY_max) ± SE of cml37, cml42, cml37 × cml42 and WT 3 and 4 day post-inoculation (dpi) with A. brassicicola spore suspension (Ab) or 0.01% Tween as mock (M). Plants shown are representative. Experiment was repeated at least two times independently [n ≥ 12 (3 dpi), n ≥ 6 (4 dpi)]. Differences in the chlorophyll fluorescence were tested by generalized least square method within the time point. Differences between the groups are indicated by different letters above the bars (p-value ≤ 0.05). Detailed information about the statistic tests and statistical values are listed in Table 3
In addition, the chlorophyll fluorescence of the leaves was measured to determine the differences in susceptibility. Corresponding to the phenotype, cml37 leaves showed lower chlorophyll fluorescence than wild type plants after fungi treatment (Fig. 5B, Table 3). Surprisingly, the chlorophyll fluorescence of cml42 was similar to those of wild type leaves (Fig. 5B, Table 3), although the necrotic area seemed to be smaller on mutant leaves (Fig. 5A). However, in cml37 × cml42 the chlorophyll fluorescence was comparable to wild type plants as well (Fig. 5B, Table 3), implying that cml42 can recover the strong negative impact of cml37. Thus, CML37 and CML42 seem to regulate the defense against A. brassicicola antagonistically as well.
Drought stress response is not altered in cml37 × cml42
An addition to their known function in jasmonate-mediated stress responses, CML37 and CML42 have been shown to regulate the drought stress response in A. thaliana (Scholz et al. 2015; Vadassery et al. 2012). It was observed that cml37 plants are more sensitive to drought stress than wild type plants, while cml42 seemed to be less affected (Scholz et al. 2015). The drought stress phenotype of both mutants was reflected in altered ABA levels: cml42 displayed higher ABA levels after drought stress, whereas in cml37 ABA was not induced at all upon drought (Scholz et al. 2015; Vadassery et al. 2012). In order to test if CML37 and CML42 are also antagonistically regulating the drought stress response, we investigated the response of cml37 × cml42 to drought. After 1 week without water, there was no difference in the drought stress phenotype between cml37 × cml42 and wild type plants (Fig. 6A). Plants were then watered and kept for a second week without water. Even after the second drought period, cml37 × cml42 was as tolerant as the wild type plants (Fig. 6A). Corresponding to that, cml37 × cml42 displayed wild type-like ABA levels among the whole treatment (Fig. 6B, Table 4). Again, the diverging effects of the single knock out mutants are balanced in the double knock out mutant line, suggesting that CML37 and CML42 are antagonists in the regulation of the drought stress response.
Drought stress responses of cml37 × cml42. A Phenotypes and B ABA contents ± SE of cml37 × cml42 and WT plants after 1 or 2 weeks of drought stress. Plants exposed to drought for 2 weeks were re-watered once after 1 week. Untreated plants were used as controls. Plants shown are representative. Experiment was repeated four times independently (n ≥ 18). ABA levels of the genotypes at 0 weeks were compared by two-sample Wilcoxon test. The influence of genotype and drought stress was tested with a two-way ANOVA (1 week) or a generalized least square method (2 weeks). Differences between the groups are indicated by the letters above the bars (p-value ≤ 0.05). For p-values see Table 4; n.s. means not significant
Discussion
The CMLs, a group of calcium sensors, has been shown to be responsible for sensing stress-mediated Ca2+ signals and regulating downstream defense reactions of the plant (Delk et al. 2005; Leba et al. 2012; Ma et al. 2008; Magnan et al. 2008; Scholz et al. 2014, 2015; Vadassery et al. 2012; Xu et al.2017; Zhu et al. 2017). However, not much is known about the interplay between the different CMLs in mediating different stress responses. Here we report about the antagonism of CML37 and CML42 in regulating the defense against herbivores and pathogens, as well as the drought stress response of A. thaliana.
CML37 and CML42 regulate the defense to S. littoralis antagonistically
In previous studies CML37 was described as a positive regulator of defense against the insect herbivore S. littoralis whereas CML42 was shown to negatively influence this defense response (Scholz et al. 2014; Vadassery et al. 2012). Here we showed that if both CMLs are knocked out, the positive effect of CML37 and the negative effect of CML42 neutralize each other, suggesting that both CMLs act antagonistically. In feeding assays, S. littoralis larvae gained as much weight on the double knock out mutant line as on wild type plants (Fig. 2). In line with this result, the double knock out mutant displayed a wild type-like phytohormone response upon S. littoralis feeding (Fig. 3). However in former studies with the corresponding single knock out mutant lines, only cml37 but not cml42 had an effect on the level of phytohormones. In detail, cml37 elevated less OPDA and JA-Ile upon insect feeding than the wild type, whereas cml42 mutants accumulated the same amounts of jasmonates like the wild type (Scholz et al. 2014; Vadassery et al. 2012). Nevertheless, cml42 is able to rescue the effect of cml37 in the double knock out mutant (Fig. 3). This result suggests that cml42 is able to positively influence the jasmonate accumulation. This positive influence of cml42 might not have been displayed in the single mutant, since enhanced jasmonate levels are usually very costly to the plants and negatively affect their fitness. Plants that exhibit higher levels of jasmonates are often smaller and produce far less seeds (Baldwin 1998; Cipollini 2007). Thus, the plant might control the level of jasmonates and limit them via other regulators to a certain level. Nevertheless, it might be possible that there are secondary effects that just appear in the double knock out mutant, but not in the single mutants that explain the different effects of knocking out CML42 in single and double knock out mutant on the jasmonate elevation.
A similar rescue effect of one of the single mutants in the double knock out line could be shown for the production of secondary metabolites. Here we investigated the levels of glucosinolates, a class of secondary metabolites produced especially as defensive compounds against herbivores in the Brassicaceae family (Halkier and Gershenzon 2006). It was known that cml42 produced higher constitutive levels of mainly aliphatic glucosinolates (Vadassery et al. 2012). In cml37 × cml42 the constitutive levels of both aliphatic and indole glucosinolates were comparable to those of wild type plants (Fig. 4B, C), suggesting that cml37 is able to counteract the effect of cml42 on the constitutive levels of glucosinolates in cml37 × cml42, although the indole glucosinolates were found to be slightly but significantly reduced after 7d of S. littoralis feeding (Fig. 4C). Thus, cml37 might negatively influence the production of glucosinolates. However, such a negative effect on glucosinolate production was not measured in the single knock out line of CML37. It displayed a wild type-like glucosinolate pattern (Scholz et al. 2014). On the other side it is known that cml37 alone negatively affected the biosynthesis of jasmonates (Scholz et al. 2014) and that the biosynthesis of glucosinolates is mainly regulated by the jasmonate pathway (Mewis et al. 2006; Schweizer et al. 2013). Thus cml37 might also negatively influence the glucosinolate production. However, even coi-1 mutants that are insensitive to jasmonates still possess a certain level of glucosinolates (Mewis et al. 2006; Schweizer et al. 2013). Clearly cml37 is impaired in the jasmonate biosynthesis, but it displays a far less severe phenotype than coi-1 (Scholz et al. 2014). Thus it is conceivable that cml37 has a negative impact on the production of glucosinolates that is not pronounced in the single mutant, but strong enough to rescue cml42 in the double knock out plants. However, also here secondary effects that occur in the double knock out mutant line cannot be fully excluded as a possible explanation of the rescue effect.
Since cml37 is able to rescue effects of cml42 and vice versa, we could clearly show that both CMLs regulate the defense against the insect herbivore S. littoralis antagonistically.
The differential regulation of defense against necrotrophs by CML37 and CML42
Besides their role in the herbivore defense, some CMLs have been shown to be involved in the defense against pathogens (Leba et al. 2012; Ma et al. 2008; Xu et al. 2017; Zhu et al. 2017). However, most of the research is focusing on the biotrophic bacterial pathogen P. syringae, but less is known about the role of CMLs in the defense against necrotrophic pathogens. In gene expression studies CML37 and CML42 were both upregulated after infection with the necrotrophic fungus Botrytis cinerea (McCormack et al. 2005). Here, we showed that CML37 and CML42 are also important for the regulation of the defense against the necrotrophic fungus A. brassicicola. Whereas cml37 was more susceptible to the fungus than the wild type plants, cml42 seemed to be more resistant (Fig. 5), indicating that CML37 acts as a positive regulator of the defense against the fungus and CML42 as a negative one. Further effects of cml37 and cml42 neutralized each other in the cml37 × cml42 double mutant (Fig. 5), providing again evidence that these two CMLs are antagonists in the regulation of this defense reaction.
The signaling pathways leading to defense against herbivores and those that are responsible for the defense against necrotrophs are closely related as both of them rely on the jasmonate pathway (Glazebrook 2005; Howe and Jander 2008; Thomma et al. 1998). Since CML37 and CML42 are influencing this phytohormone pathway [Scholz et al. (2014); Vadassery et al. (2012) and Fig. 3], it is not surprising that they have similar roles in the regulation of the defense against the herbivore S. littoralis and the pathogen A. brassicicola.
The antagonism of CML37 and CML42 in the drought stress response
Besides biotic stress, plants have to cope with changing abiotic conditions. The availability of water is one of the major abiotic factors. It is known that CMLs play important roles in regulating the drought stress response. ShCML44, a CML isolated from wild tomato plants (Solanum habrochaites), confers to drought tolerance when overexpressed in Arabidopsis (Munir et al. 2016). The same effect was shown for the rice (Oryza sativa) CML OsMSR2 (Xu et al. 2011). Also CML37 and CML42 have been shown to mediate the drought stress response of Arabidopsis, whereby CML37 was revealed as a positive regulator and CML42 as a negative regulator (Scholz et al. 2015; Vadassery et al. 2012). By studying the drought stress response of cml37 × cml42, we could show that CML37 and CML42 are also antagonists in the regulation of the drought stress response. Similar to the response to biotic stress treatments, cml37 × cml42 displayed a wild type-like drought stress phenotype (Fig. 6A), suggesting that the opposite effects found in cml37 and cml42 neutralize each other in cml37 × cml42.
The response to abiotic stresses is mainly regulated by the phytohormone ABA (Vishwakarma et al. 2017). Both cml37 and cml42 displayed altered ABA levels upon drought stress. In cml42 levels of ABA were increased compared to wild type plants, whereas in cml37 ABA was not induced at all after drought treatments (Scholz et al. 2015; Vadassery et al. 2012). In line with the drought stress phenotype of cml37 × cml42, plants elevated as much ABA upon drought as wild type plants, confirming the thesis that CML37 and CML42 regulate the drought stress response antagonistically.
The function of the CML37/CML42 antagonism in plant stress regulation
By investigating a cml37 × cml42 double knock out line, we could show that the two Ca2+ sensors CML37 and CML42 act antagonistically in both the jasmonate-mediated regulation of biotic as well as ABA-mediated regulation of abiotic stress responses. Whereas CML37 performs in all cases as a positive regulator of the stress responses, CML42 is counteracting as a negative regulator. For the Ca2+ binding proteins EHB1 and AGD12, in Arabidopsis reduced phototropism and gravitropism in agd12 mutants but enhanced phototropism and gravitropism in ehb1 mutants was demonstrated (Michalski et al. 2017; Dümmer et al. 2016).
Strikingly, in the cml37 × cml42 plants both single effects are balanced out, generating again a wild type-like phenotype (summarized in Fig. 7). Similar effects have been shown for two zinc finger proteins in Arabidopsis, LSD1 and LOL1, which antagonistically regulate pathogen-induced cell death: A double knock out of both proteins led to a wild type-like cell death response (Epple et al. 2003). It was hypothesized by the authors that this antagonism is used in the plant to control the cell death response: an imbalance towards the positive regulator would activate the cell death response, whereas an imbalance towards the negative regulator would counteract it (Epple et al. 2003). Similarly, also CML37 and CML42 could fine tune defense responses in the plant. This can be used by the plant also to coordinate competing responses to stresses that occur at the same time. However, further studies dealing with parallel stress treatments are needed to address this hypothesis.
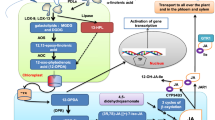
Model for the antagonistic effects of CML37 and CML42 on different stress responses in Arabidopsis. CML37 and CML42 are Ca2+ sensors that act antagonistically on the plant stress response to drought, herbivores and necrotrophs. After sensing Ca2+ elevations in the cell, they bind to yet unknown targets [CML37/CML42 interacting protein(s)] and influence downstream phytohormone pathways. CML37 positively influences the ABA elevation induced by drought stress, enhancing drought tolerance. CML42 negatively affects the drought-induced ABA elevation, and thus the drought tolerance. CML37 has a positive effect on the jasmonate biosynthesis that is counteracted by CML42. CML42 negatively influences jasmonate-dependent responses. Thus CML37 positively influences the defense against herbivores, whereas CML42 has a negative impact. Further CML37 and CML42 play the same roles in the defense against necrotrophic fungi, which might be connected to their effects on the jasmonate signaling pathway. In all shown defense reactions positive and negative effects of CML37 and CML42 are balanced out. CML37 and CML42 might be used for fine tuning these stress responses. Symbols: + means a positive influence,—a negative influence, dashed lines indicate that the influence was proven indirectly by examining the double knock out line of CML37 and CML42
Further, CMLs belong to the class of Ca2+ sensor relays and thus just possess the functional domains to bind Ca2+ (Sanders et al. 2002). Therefore, to transduce the information given in the Ca2+ signature into the appropriate defense response, they need to bind to a certain target protein. For instance, it was shown that the soybean (Glycine max) GmCaM1 and GmCML1 (former GmCaM4) antagonistically regulate the activity of the transcription factor MYB2 in Arabidopsis (Yoo et al. 2005) and, thus, the salt stress response. Similarly, CML37 and CML42 might influence transcription factors that are known to control the expression of jasmonate or ABA responsive genes. For example, Danisman et al. (2012) showed the antagonistic function of class I and class II TCP transcription factors in the control of leaf development. Furthermore CML37 and CML42 might form heterodimers leading to the antagonistic effects as it known from the above mentioned LSD1 and LOL1 (Epple et al. 2003). To understand the antagonism between the two CMLs in detail, the downstream interacting proteins need to be identified in future projects.
Data availability
Data available upon request.
References
Baldwin IT (1998) Jasmonate-induced responses are costly but benefit plants under attack in native populations. Proc Natl Acad Sci USA 95:8113–8118. https://doi.org/10.1073/pnas.95.14.8113
Bender KW, Snedden WA (2013) Calmodulin-related proteins step out from the shadow of their namesake. Plant Physiol 163:486–495. https://doi.org/10.1104/pp.113.221069
Bergomaz R, Boppré M (1986) A simple instant diet for rearing arctiidae and other moths. J Lepid Soc 40:131–137
Burow M, Müller R, Gershenzon J, Wittstock U (2006) Altered glucosinolate hydrolysis in genetically engineered Arabidopsis thaliana and its influence on the larval development of Spodoptera littoralis. J Chem Ecol 32:2333–2349. https://doi.org/10.1007/s10886-006-9149-1
Cipollini D (2007) Consequences of the overproduction of methyl jasmonate on seed production, tolerance to defoliation and competitive effect and response of Arabidopsis thaliana. New Phytol 173:146–153. https://doi.org/10.1111/j.1469-8137.2006.01882
Crawley MJ (2013) The R book, 2nd edn. Wiley, Chicester
Danisman S, van der Wal F, Dhondt S, Waites R, de Folter S, Bimbo A, van Dijk ADJ, Muino JM, Cutri L, Dornelas MC, Angenent GC, Imminck RGH (2012) Arabidopsis class I and class II TCP transcription factors regulate jasmonic acid metabolism and leaf development antagonistically. Plant Physiol 159:1511–1523. https://doi.org/10.1104/pp.112.200303
Delk NA, Johnson KA, Chowdhury NI, Braam J (2005) CML24, regulated in expression by diverse stimuli, encodes a potential Ca2+ sensor that functions in responses to abscisic acid, daylength, and ion stress. Plant Physiol 139:240–253. https://doi.org/10.1104/pp.105.062612
Dobney S, Chiasson D, Lam P, Smith SP, Snedden WA (2009) The calmodulin-related calcium sensor CML42 plays a role in trichome branching. J Biol Chem 284:31647–31657. https://doi.org/10.1074/jbc.M109.056770
Dümmer M, Michalski C, Essen L-O, Rath M, Galland P, Forreiter C (2016) EHB1 and AGD12, two calcium-dependent proteins affect gravitropism antagonistically in Arabidopsis thaliana. J Plant Physiol 206:114–124. https://doi.org/10.1016/j.jplph.2016.09.006
Ehrhardt DW, Wais R, Long SR (1996) Calcium spiking in plant root hairs responding to Rhizobium nodulation signals. Cell 85:673–681. https://doi.org/10.1016/S0092-8674(00)81234-9
Epple P, Mack AA, Morris VRF, Dangl JL (2003) Antagonistic control of oxidative stress-induced cell death in Arabidopsis by two related, plant-specific zinc finger proteins. Proc Natl Acad Sci USA 100:6831–6836. https://doi.org/10.1073/pnas.1130421100
Fonseca S, Chini A, Hamberg M, Adie B, Porzel A, Kramell R, Miersch O, Wasternack C, Solano R (2009) (+)-7-iso-Jasmonoyl-L-isoleucine is the endogenous bioactive jasmonate. Nat Chem Biol 5:344–350. https://doi.org/10.1038/nchembio.161
Glazebrook J (2005) Contrasting mechanisms of defense against biotrophic and necrotrophic pathogens. Annu Rev Phytopathol 43:205–227. https://doi.org/10.1146/annurev.phyto.43.040204.135923
Graser G, Schneider B, Oldham NJ, Gershenzon J (2000) The methionine chain elongation pathway in the biosynthesis of glucosinolates in Eruca sativa (Brassicaceae). Arch Biochem Biophys 378:411–419. https://doi.org/10.1006/abbi.2000.1812
Halkier BA, Gershenzon J (2006) Biology and biochemistry of glucosinolates. Annu Rev Plant Biol 57:303–333. https://doi.org/10.1146/annurev.arplant.57.032905.105228
Heyer M, Scholz SS, Voigt D, Reichelt M, Aldon D, Oelmüller R, Boland W, Mithöfer A (2018) Herbivory-responsive calmodulin-like protein CML9 does not guide jasmonate-mediated defenses in Arabidopsis thaliana. PLoS ONE 13:e0197633. https://doi.org/10.1371/journal.pone.0197633
Howe GA, Jander G (2008) Plant immunity to insect herbivores. Annu Rev Plant Biol 59:41–66. https://doi.org/10.1146/annurev.arplant.59.032607.092825
Jimenez-Aleman GH, Scholz SS, Heyer M, Reichelt M, Mithöfer A, Boland W (2015) Synthesis, metabolism and systemic transport of a fluorinated mimic of the endogenous jasmonate precursor OPC-8:0. Biochim Biophys Acta Mol Cell Biol Lipids 1851:1545–1553. https://doi.org/10.1016/j.bbalip.2015.09.002
Johnson JM, Sherameti I, Nongbri PL, Oelmüller R (2013) Standardized conditions to study beneficial and nonbeneficial traits in the Piriformospora indica/Arabidopsis thaliana Interaction. In: Varma A, Kost G, Oelmüller R (eds) Piriformospora indica: sebacinales and their biotechnological applications. Springer, Berlin, pp 325–343
Knight MR, Campbell AK, Smith SM, Trewavas AJ (1991) Transgenic plant aequorin reports the effects of touch and cold-shock and elicitors on cytoplasmic calcium. Nature 352:524–526. https://doi.org/10.1038/352524a0
Knight H, Trewavas AJ, Knight MR (1997) Calcium signalling in Arabidopsis thaliana responding to drought and salinity. Plant J 12:1067–1078. https://doi.org/10.1046/j.1365-313X.1997.12051067
Konieczny A, Ausubel FM (1993) A procedure for mapping Arabidopsis mutations using co-dominant ecotype-specific PCR-based markers. Plant J 4:403–410. https://doi.org/10.1046/j.1365-313X.1993.04020403
Kramell R, Schmidt J, Schneider G, Sembdner G, Schreiber K (1988) Synthesis of N-(jasmonoyl)amino acid conjugates. Tetrahedron 44:5791–5807. https://doi.org/10.1016/S0040-4020(01)81437
Kretsinger RH, Nockolds CE (1973) Carp muscle calcium-binding protein: II. Structure determination and general description. J Biol Chem 248:3313–3326. https://doi.org/10.1016/S0021-9258(19)44043-X
Leba LJ, Cheval C, Ortiz-Martin I, Ranty B, Beuzon CR, Galaud JP, Aldon D (2012) CML9, an Arabidopsis calmodulin-like protein, contributes to plant innate immunity through a flagellin-dependent signalling pathway. Plant J 71:976–989. https://doi.org/10.1111/j.1365-313X.2012.05045
Ma W, Smigel A, Tsai Y-C, Braam J, Berkowitz GA (2008) Innate immunity signaling: cytosolic Ca2+ elevation is linked to downstream nitric oxide generation through the action of calmodulin or a calmodulin-like protein. Plant Physiol 148:818–828. https://doi.org/10.1104/pp.108.125104
Maffei M, Bossi S, Spiteller D, Mithöfer A, Boland W (2004) Effects of feeding Spodoptera littoralis on lima bean leaves. I. Membrane potentials, intracellular calcium variations, oral secretions, and regurgitate components. Plant Physiol 134:1752–1762. https://doi.org/10.1104/pp.103.034165
Magnan F, Ranty B, Charpenteau M, Sotta B, Galaud JP, Aldon D (2008) Mutations in AtCML9, a calmodulin-like protein from Arabidopsis thaliana, alter plant responses to abiotic stress and abscisic acid. Plant J 56:575–589. https://doi.org/10.1111/j.1365-313X.2008.03622
McAinsh MR, Brownlee C, Hetherington AM (1997) Calcium ions as second messengers in guard cell signal transduction. Physiol Plant 100:16–29. https://doi.org/10.1111/j.1399-3054.1997.tb03451
McCormack E, Tsai YC, Braam J (2005) Handling calcium signaling: Arabidopsis CaMs and CMLs. Trends Plant Sci 10:383–389. https://doi.org/10.1016/j.tplants.2005.07.001
Mewis I, Tokuhisa JG, Schultz JC, Appel HM, Ulrichs C, Gershenzon J (2006) Gene expression and glucosinolate accumulation in Arabidopsis thaliana in response to generalist and specialist herbivores of different feeding guilds and the role of defense signaling pathways. Phytochemistry 67:2450–2462. https://doi.org/10.1016/j.phytochem.2006.09.004
Michalski C, Dümmer M, Galland P, Forreiter C (2017) Impact of EHB1 and AGD12 on root and hypocotyl phototropism in Arabidopsis thaliana. J Plant Growth Regul 36:660–668. https://doi.org/10.1007/s00344-017-9667-9
Munir S, Liu H, Xing HS, Ouyang B, Zhang Y, Li H, Ye Z (2016) Overexpression of calmodulin-like (ShCML44) stress-responsive gene from Solanum habrochaites enhances tolerance to multiple abiotic stresses. Sci Rep 6:1–10. https://doi.org/10.1038/srep31772
Pinheiro J, Bates D, DebRoy S, Sarkar D, R Core Team (2018) nlme: linear and nonlinear mixed effects models. R package version 3.1–137. https://CRAN.R-project.org/package=nlme
Plieth C (2016) Calcium, metaphors, and zeitgeist in plant sciences. Plant Physiol 171:1790–1793. https://doi.org/10.1104/pp.16.00645
Plieth C, Hansen UP, Knight H, Knight MR (1999) Temperature sensing by plants: the primary characteristics of signal perception and calcium response. Plant J 18:491–497. https://doi.org/10.1046/j.1365-313X.1999.00471
R Development Core Team (2018) R: a language and environment for statistical computing. R Foundation for statistical computing, Vienna, Austria. http://www.R-project.org
Sanders D, Pelloux J, Brownlee C, Harper JF (2002) Calcium at the crossroads of signaling. Plant Cell 14:S401–S417. https://doi.org/10.1105/tpc.002899
Scholz SS, Vadassery J, Heyer M, Reichelt M, Bender KW, Snedden WA, Boland W, Mithöfer A (2014) Mutation of the Arabidopsis calmodulin-like protein CML37 deregulates the jasmonate pathway and enhances susceptibility to herbivory. Mol Plant 7:1712–1726. https://doi.org/10.1093/mp/ssu102
Scholz SS, Reichelt M, Vadassery J, Mithöfer A (2015) Calmodulin-like protein CML37 is a positive regulator of ABA during drought stress in Arabidopsis. Plant Signal Behav 10:e1011951. https://doi.org/10.1080/15592324.2015.1011951
Schweizer F, Fernández-Calvo P, Zander M, Diez-Diaz M, Fonseca S, Glauser G, Lewsey MG, Ecker JR, Solano R, Reymond P (2013) Arabidopsis basic helix-loop-helix transcription factors MYC2, MYC3, and MYC4 regulate glucosinolate biosynthesis, insect performance, and feeding behavior. Plant Cell 25:3117–3132. https://doi.org/10.1105/tpc.113.115139
Shabab M, Khan SA, Vogel H, Heckel DG, Boland W (2014) OPDA isomerase GST16 is involved in phytohormone detoxification and insect development. FEBS J 281:2769–2783. https://doi.org/10.1111/febs.12819
Shacklock PS, Read ND, Trewavas AJ (1992) Cytosolic free calcium mediates red light-induced photomorphogenesis. Nature 358:753–755. https://doi.org/10.1038/358753a0
Systat Software (2008) SigmaPlot. San Jose California, USA. www.systatsoftware.com
Thies W (1988) Isolation of sinigrin and glucotropaeolin from cruciferous seeds. Eur J Lipid Sci Technol 90:311–314. https://doi.org/10.1002/lipi.19880900806
Thomma BPHJ, Eggermont K, Penninckx IAMA, Mauch-Mani B, Vogelsang R, Cammue BPA, Broekaert WF (1998) Separate jasmonate-dependent and salicylate-dependent defense-response pathways in Arabidopsis are essential for resistance to distinct microbial pathogens. Proc Natl Acad Sci USA 95:15107–15111. https://doi.org/10.1073/pnas.95.25.15107
Vadassery J, Ranf S, Drzewiecki C, Mithöfer A, Mazars C, Scheel D, Lee J, Oelmüller R (2009) A cell wall extract from the endophytic fungus Piriformospora indica promotes growth of Arabidopsis seedlings and induces intracellular calcium elevation in roots. Plant J 59:193–206. https://doi.org/10.1111/j.1365-313X.2009.03867.x
Vadassery J, Reichelt M, Hause B, Gershenzon J, Boland W, Mithöfer A (2012) CML42-mediated calcium signaling coordinates responses to Spodoptera herbivory and abiotic stresses in Arabidopsis. Plant Physiol 159:1159–1175. https://doi.org/10.1104/pp.112.198150
Vishwakarma K, Upadhyay N, Kumar N, Yadav G, Singh J, Mishra RK, Kumar V, Verma R, Upadhyay RG, Pandey M, Sharma S (2017) Abscisic acid signaling and abiotic stress tolerance in plants: a review on current knowledge and future prospects. Front Plant Sci 8:1–12. https://doi.org/10.3389/fpls.2017.00161
Xu GY, Rocha PS, Wang ML, Xu ML, Cui YC, Li LY, Zhu YX, Xia X (2011) A novel rice calmodulin-like gene, OsMSR2, enhances drought and salt tolerance and increases ABA sensitivity in Arabidopsis. Planta 234:47–59. https://doi.org/10.1007/s00425-011-1386
Xu B, Cheval C, Laohavisit A, Hocking B, Chiasson D, Olsson TSG, Shirasu K, Faulkner C, Gilliham M (2017) A calmodulin-like protein regulates plasmodesmal closure during bacterial immune responses. New Phytol 215:77–84. https://doi.org/10.1111/nph.14599
Yoo JH, Park CY, Kim JC, Heo WD, Cheong MS, Park HC, Kim MC, Moon BC, Choi MS, Kang YH, Lee JH, Kim HS, Lee SM, Yoon HW, Lim CO, Yun DJ, Lee SY, Chung WS, Cho MJ (2005) Direct interaction of a divergent CaM isoform and the transcription factor, MYB2, enhances salt tolerance in Arabidopsis. J Biol Chem 280:3697–3706. https://doi.org/10.1074/jbc.M408237200
Zhu X, Robe E, Jomat L, Aldon D, Mazars C, Galaud J-P (2017) CML8, an Arabidopsis calmodulin-like protein, plays a role in Pseudomonas syringae Plant immunity. Plant Cell Physiol 58:307–319. https://doi.org/10.1093/pcp/pcw189
Zuur AF, Ieno EN, Walker N, Saveliev AA, Smith GM (2009) Mixed effects models and extensions in ecology with R. Springer, New York
Acknowledgements
We thank Sandra Fonseca for kindly providing the entry clones of JAR1. We thank Andrea Lehr and Angelika Berg for rearing S. littoralis larvae and the MPI greenhouse team for growing Arabidopsis plants. We thank the Max Planck Society for funding. Sandra S. Scholz and Ralf Oelmüller were supported by the Deutsche Forschungsgemenschaft (Grant No. CRC1127).
Funding
Open Access funding enabled and organized by Projekt DEAL. The current work was funded by the Max Planck Society and the Deutsche Forschungsgemeinschaft (Grant No. CRC1127).
Author information
Authors and Affiliations
Contributions
MH and AM designed the experiments. MH, SSS, MR performed experiments. MH, GK did the statistical analyses; MH, SSS, MR, GK, RO, AM analyzed the data. MH and AM wrote the manuscript with contribution of all other authors.
Corresponding author
Ethics declarations
Conflict of interest
The authors declare no conflicts of interest.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/.
About this article
Cite this article
Heyer, M., Scholz, S.S., Reichelt, M. et al. The Ca2+ sensor proteins CML37 and CML42 antagonistically regulate plant stress responses by altering phytohormone signals. Plant Mol Biol 109, 611–625 (2022). https://doi.org/10.1007/s11103-021-01184-2
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11103-021-01184-2