Abstract
Key message
YGL8 has the dual functions in Chl biosynthesis: one as a catalytic subunit of MgPME cyclase, the other as a core component of FLU-YGL8-LCAA-POR complex in Chl biosynthesis.
Abstract
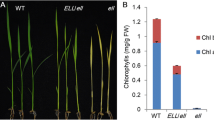
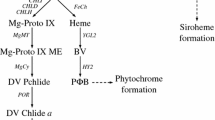
Magnesium-protoporphyrin IX monomethyl ester (MgPME) cyclase is an essential enzyme involved in chlorophyll (Chl) biosynthesis. However, its roles in regulating Chl biosynthesis are not fully explored. In this study, we isolated a rice mutant yellow-green leaf 8 (ygl8) that exhibited chlorosis phenotype with abnormal chloroplast development in young leaves. As the development of leaves, the chlorotic plants turned green accompanied by restorations in Chl content and chloroplast ultrastructure. Map-based cloning revealed that the ygl8 gene encodes a catalytic subunit of MgPME cyclase. The ygl8 mutation caused a conserved amino acid substitution (Asn182Ser), which was related to the alterations of Chl precursor content. YGL8 was constitutively expressed in various tissues, with more abundance in young leaves and panicles. Furthermore, we showed that expression levels of some nuclear genes associated with Chl biosynthesis were affected in both the ygl8 mutant and YGL8 RNA interference lines. By transient expression in rice protoplasts, we found that N-terminal 40 amino acid residues were enough to localize the YGL8 protein to chloroplast. In vivo experiments demonstrated a physical interaction between YGL8 and a rice chloroplast protein, low chlorophyll accumulation A (OsLCAA). Moreover, bimolecular fluorescence complementation assays revealed that YGL8 also interacted with the other two rice chloroplast proteins, viz. fluorescent (OsFLU1) and NADPH:protochlorophyllide oxidoreductase (OsPORB). These results provide new insights into the roles of YGL8, not only as a subunit with catalytic activity, but as a core component of FLU–YGL8–LCAA–POR complex required for Chl biosynthesis.










Similar content being viewed by others
References
Albus CA, Salinas A, Czarnecki O, Kahlau S, Rothbart M, Thiele W, Lein W, Bock R, Grimm B, Schottler MA (2012) LCAA, a novel factor required for magnesium protoporphyrin monomethylester cyclase accumulation and feedback control of aminolevulinic acid biosynthesis in tobacco. Plant Physiol 160:1923–1939
Arnon DI (1949) Copper enzymes in isolated chloroplasts: polyphenoloxidase in Beta vulgaris. Plant Physiol 24:1–15
Bang WY, Jeong IS, Kim DW, Im CH, Ji C, Hwang SM, Kim SW, Son YS, Jeong J, Shiina T, Bahk JD (2008) Role of Arabidopsis CHL27 protein for photosynthesis, chloroplast development and gene expression profiling. Plant Cell Physiol 49:1350–1363
Berthold DA, Stenmark P (2003) Membrane-bound diiron carboxylate proteins. Annu Rev Plant Biol 54:497–517
Bollivar D, Braumann I, Berendt K, Gough SP, Hansson M (2014) The Ycf54 protein is part of the membrane component of Mg-protoporphyrin IX monomethyl ester cyclase from barley (Hordeum vulgare L.) FEBS J 281:2377–2386
Bruce BD (2000) Chloroplast transit peptides: structure, function and evolution. Trends Cell Biol 10:440–447
Brzezowski P, Richter AS, Grimm B (2015) Regulation and function of tetrapyrrole biosynthesis in plants and algae. Biochim Biophys Acta 1847:968–985
Chen H, Cheng Z, Ma X, Wu H, Liu Y, Zhou K, Chen Y, Ma W, Bi J, Zhang X, Guo X, Wang J, Lei C, Wu F, Lin Q, Liu Y, Liu L, Jiang L (2013) A knockdown mutation of yellow-green leaf 2 blocks chlorophyll biosynthesis in rice. Plant Cell Rep 32:1855–1867
Czarnecki O, Hedtke B, Melzer M, Rothbart M, Richter A, Schroter Y, Pfannschmidt T, Grimm B (2011) An Arabidopsis GluTR binding protein mediates spatial separation of 5-aminolevulinic acid synthesis in chloroplasts. Plant Cell 23:4476–4491
Deng XJ, Zhang HQ, Wang Y, He F, Liu JL, Xiao X, Shu ZF, Li W, Wang GH, Wang GL (2014) Mapped clone and functional analysis of leaf-color gene Ygl7 in a rice hybrid (Oryza sativa L. ssp. indica). PLoS One 9:e99564
Goslings D, Meskauskiene R, Kim C, Lee KP, Nater M, Apel K (2004) Concurrent interactions of heme and FLU with Glu tRNA reductase (HEMA1), the target of metabolic feedback inhibition of tetrapyrrole biosynthesis, in dark- and light-grown Arabidopsis plants. Plant J 40:957–967
Hiei Y, Ohta S, Komari T, Kumashiro T (1994) Efficient transformation of rice (Oryza sativa L.) mediated by Agrobacterium and sequence analysis of the boundaries of the T-DNA. Plant J 6:271–282
Hollingshead S, Kopecna J, Jackson PJ, Canniffe DP, Davison PA, Dickman MJ, Sobotka R, Hunter CN (2012) Conserved chloroplast open-reading frame ycf54 is required for activity of the magnesium protoporphyrin monomethylester oxidative cyclase in Synechocystis PCC 6803. J Biol Chem 287:27823–27833
Hung CY, Sun YH, Chen J, Darlington DE, Williams AL, Burkey KO, Xie J (2010) Identification of a Mg-protoporphyrin IX monomethyl ester cyclase homologue, EaZIP, differentially expressed in variegated Epipremnum aureum ‘Golden Pothos’ is achieved through a unique method of comparative study using tissue regenerated plants. J Exp Bot 61:1483–1493
Kauss D, Bischof S, Steiner S, Apel K, Meskauskiene R (2012) FLU, a negative feedback regulator of tetrapyrrole biosynthesis, is physically linked to the final steps of the Mg++-branch of this pathway. FEBS Lett 586:211–216
Kleine T, Voigt C, Leister D (2009) Plastid signalling to the nucleus: messengers still lost in the mists? Trends Genet 25:185–192
Larkin RM, Alonso JM, Ecker JR, Chory J (2003) GUN4, a regulator of chlorophyll synthesis and intracellular signaling. Science 299:902–906
Lee S, Kim JH, Yoo ES, Lee CH, Hirochika H, An G (2005) Differential regulation of chlorophyll a oxygenase genes in rice. Plant Mol Biol 57:805–818
Lee KP, Kim C, Landgraf F and Apel K (2007) EXECUTER1- and EXECUTER2-dependent transfer of stress-related signals from the plastid to the nucleus of Arabidopsis thaliana. Proc Natl Acad Sci USA 104:10270–10275
Lichtenthaler HK (1987) Chlorophylls and carotenoids: pigments of photosynthetic biomembranes. Methods Enzymol 148:350–382
Li Q, Zhu FY, Gao X, Sun Y, Li S, Tao Y, Lo C, Liu H (2014) Young Leaf Chlorosis 2 encodes the stroma-localized heme oxygenase 2 which is required for normal tetrapyrrole biosynthesis in rice. Planta 240:701–712
Liu N, Yang YT, Liu HH, Yang GD, Zhang NH, Zheng CC (2004) NTZIP antisense plants show reduced chlorophyll levels. Plant Physiol Biochem 42:321–327
Livak KJ, Schmittgen TD (2001) Analysis of relative gene expression data using real-time quantitative PCR and the 2−∆∆CT method. Methods 25:402–408
Luo T, Fan T, Liu Y, Rothbart M, Yu J, Zhou S, Grimm B, Luo M (2012) Thioredoxin redox regulates ATPase activity of magnesium chelatase CHLI subunit and modulates redox-mediated signaling in tetrapyrrole biosynthesis and homeostasis of reactive oxygen species in pea plants. Plant Physiol 159:118–130
Masuda T, Fujita Y (2008) Regulation and evolution of chlorophyll metabolism. Photochem Photobiol Sci 7:1131–1149
Meskauskiene R, Apel K (2002) Interaction of FLU, a negative regulator of tetrapyrrole biosynthesis, with the glutamyl-tRNA reductase requires the tetratricopeptide repeat domain of FLU. FEBS Lett 532:27–30
Moseley J, Quinn J, Eriksson M, Merchant S (2000) The Crd1 gene encodes a putative di-iron enzyme required for photosystem I accumulation in copper deficiency and hypoxia in Chlamydomonas reinhardtii. EMBO J 19:2139–2151
Moseley JL, Page MD, Alder NP, Eriksson M, Quinn J, Soto F, Theg SM, Hippler M, Merchant S (2002) Reciprocal expression of two candidate di-iron enzymes affecting photosystem I and light-harvesting complex accumulation. Plant Cell 14:673–688
Moser J, Schubert WD, Beier V, Bringemeier I, Jahn D, Heinz DW (2001) V-shaped structure of glutamyl-tRNA reductase, the first enzyme of tRNA-dependent tetrapyrrole biosynthesis. EMBO J 20:6583–6590
Nogaj LA, Beale SI (2005) Physical and kinetic interactions between glutamyl-tRNA reductase and glutamate-1-semialdehyde aminotransferase of Chlamydomonas reinhardtii. J Biol Chem 280:24301–24307
Ouchane S, Steunou AS, Picaud M, Astier C (2004) Aerobic and anaerobic Mg-protoporphyrin monomethyl ester cyclases in purple bacteria: a strategy adopted to bypass the repressive oxygen control system. J Biol Chem 279:6385–6394
Pérez-Ruiz JM, Guinea M, Puerto-Galán L, Cejudo FJ (2014) NADPH thioredoxin reductase C is involved in redox regulation of the Mg-chelatase I subunit in Arabidopsis thaliana chloroplasts. Mol Plant 7:1252–1255
Peter E, Salinas A, Wallner T, Jeske D, Dienst D, Wilde A, Grimm B (2009) Differential requirement of two homologous proteins encoded by sll1214 and sll1874 for the reaction of Mg protoporphyrin monomethylester oxidative cyclase under aerobic and micro-oxic growth conditions. Biochim Biophys Acta 1787:1458–1467
Peter E, Rothbart M, Oelze ML, Shalygo N, Dietz KJ, Grimm B (2010) Mg protoporphyrin monomethylester cyclase deficiency and effects on tetrapyrrole metabolism in different light conditions. Plant Cell Physiol 51:1229–1241
Pinta V, Picaud M, Reiss-Husson F, Astier C (2002) Rubrivivax gelatinosus acsF (previously orf358) codes for a conserved, putative binuclear-iron-cluster-containing protein involved in aerobic oxidative cyclization of Mg-protoporphyrin IX monomethylester. J Bacteriol 184:746–753
Rebeiz CA, Mattheis JR, Smith BB, Rebeiz CC, Dayton DF (1975) Chloroplast biogenesis: biosynthesis and accumulation of protochlorophyll by isolated etioplasts and developing chloroplasts. Arch Biochem Biophys 171:549–567
Richter A, Peter E, Pors Y, Lorenzen S, Grimm B, Czarnecki O (2010) Rapid dark repression of 5-aminolevulinic acid synthesis in green barley leaves. Plant Cell Physiol 51:670–681
Richter AS, Peter E, Rothbart M, Schlicke H, Toivola J, Rintamaki E, Grimm B (2013) Posttranslational influence of NADPH-dependent thioredoxin reductase C on enzymes in tetrapyrrole synthesis. Plant Physiol 162:63–73
Rzeznicka K, Walker CJ, Westergren T, Kannangara CG, von Wettstein D, Merchant S, Gough SP, Hansson M (2005) Xantha-l encodes a membrane subunit of the aerobic Mg-protoporphyrin IX monomethyl ester cyclase involved in chlorophyll biosynthesis. Proc Natl Acad Sci USA 102:5886–5891
Sakuraba Y, Rahman ML, Cho SH, Kim YS, Koh HJ, Yoo SC, Paek NC (2013) The rice faded green leaf locus encodes protochlorophyllide oxidoreductase B and is essential for chlorophyll synthesis under high light conditions. Plant J 74:122–133
Stenbaek A, Hansson A, Wulff RP, Hansson M, Dietz KJ, Jensen PE (2008) NADPH-dependent thioredoxin reductase and 2-Cys peroxiredoxins are needed for the protection of Mg-protoporphyrin monomethyl ester cyclase. FEBS Lett 582:2773–2778
Stoutjesdijk PA, Singh SP, Liu Q, Hurlstone CJ, Waterhouse PA, Green AG (2002) hpRNA-mediated targeting of the Arabidopsis FAD2 gene gives highly efficient and stable silencing. Plant Physiol 129:1723–1731
Strand A, Asami T, Alonso J, Ecker JR, Chory J (2003) Chloroplast to nucleus communication triggered by accumulation of Mg-protoporphyrin IX. Nature 421:79–83
Tanaka R, Tanaka A (2007) Tetrapyrrole biosynthesis in higher plants. Annu Rev Plant Biol 58:321–346
Terry MJ, Smith AG (2013) A model for tetrapyrrole synthesis as the primary mechanism for plastid-to-nucleus signaling during chloroplast biogenesis. Front Plant Sci 4:14
Thompson JD, Higgins DG, Gibson TJ (1994) CLUSTAL W: improving the sensitivity of progressive multiple sequence alignment through sequence weighting, position-specific gap penalties and weight matrix choice. Nucleic Acids Res 22:4673–4680
Tottey S, Block MA, Allen M, Westergren T, Albrieux C, Scheller HV, Merchant S, Jensen PE (2003) Arabidopsis CHL27, located in both envelope and thylakoid membranes, is required for the synthesis of protochlorophyllide. Proc Natl Acad Sci USA 100:16119–16124
Waadt R and Kudla J (2008) In planta visualization of protein interactions using bimolecular fluorescence complementation (BiFC). CSH Protoc doi:10.1101/pdb.prot4995
Wallar BJ, Lipscomb JD (1996) Dioxygen activation by enzymes containing binuclear non-heme iron clusters. Chem Rev 96:2625–2658
Wang P, Grimm B (2015) Organization of chlorophyll biosynthesis and insertion of chlorophyll into the chlorophyll-binding proteins in chloroplasts. Photosynth Res 126:189–202
Wang P, Gao J, Wan C, Zhang F, Xu Z, Huang X, Sun X, Deng X (2010) Divinyl chlorophyll(ide) a can be converted to monovinyl chlorophyll(ide) a by a divinyl reductase in rice. Plant Physiol 153:994–1003
Wong YS, Castelfranco PA (1984) Resolution and reconstitution of Mg-protoporphyrin IX monomethyl ester (oxidative) cyclase, the enzyme system responsible for the formation of the chlorophyll isocyclic ring. Plant Physiol 75:658–661
Wu Z, Zhang X, He B, Diao L, Sheng S, Wang J, Guo X, Su N, Wang L, Jiang L, Wang C, Zhai H, Wan J (2007) A chlorophyll-deficient rice mutant with impaired chlorophyllide esterification in chlorophyll biosynthesis. Plant Physiol 145:29–40
Zhang H, Li J, Yoo JH, Yoo SC, Cho SH, Koh HJ, Seo HS, Paek NC (2006) Rice Chlorina-1 and Chlorina-9 encode ChlD and ChlI subunits of Mg-chelatase, a key enzyme for chlorophyll synthesis and chloroplast development. Plant Mol Biol 62:325–337
Zhang Y, Su J, Duan S, Ao Y, Dai J, Liu J, Wang P, Li Y, Liu B, Feng D, Wang J, Wang H (2011) A highly efficient rice green tissue protoplast system for transient gene expression and studying light/chloroplast-related processes. Plant Methods 7:30
Zhang M, Zhang F, Fang Y, Chen X, Chen Y, Zhang W, Dai HE, Lin R, Liu L (2015) The non-canonical tetratricopeptide repeat (TPR) domain of Fluorescent (FLU) mediates complex formation with glutamyl-tRNA reductase. J Biol Chem 290:17559–17565
Zheng CC, Porat R, Lu P, O’Neill SD (1998) PNZIP is a novel mesophyll-specific cDNA that is regulated by phytochrome and the circadian rhythm and encodes a protein with a leucine zipper motif. Plant Physiol 116:27–35
Zhou S, Wang Y, Li W, Zhao Z, Ren Y, Wang Y, Gu S, Lin Q, Wang D, Jiang L, Su N, Zhang X, Liu L, Cheng Z, Lei C, Wang J, Guo X, Wu F, Ikehashi H, Wang H, Wan J (2011) Pollen semi-sterility1 encodes a kinesin-1-like protein important for male meiosis, anther dehiscence, and fertility in rice. Plant Cell 23:111–129
Zhou S, Sawicki A, Willows RD, Luo M (2012) C-terminal residues of Oryza sativa GUN4 are required for the activation of the ChlH subunit of magnesium chelatase in chlorophyll synthesis. FEBS Lett 586:205–210
Zhou K, Ren Y, Lv J, Wang Y, Liu F, Zhou F, Zhao S, Chen S, Peng C, Zhang X, Guo X, Cheng Z, Wang J, Wu F, Jiang L, Wan J (2013) Young leaf chlorosis 1, a chloroplast-localized gene required for chlorophyll and lutein accumulation during early leaf development in rice. Planta 237:279–292
Acknowledgments
This work was supported by the grants from the National Transform Science and Technology Program (2013ZX08001004-002), the Chinese National High Technology Research and Development Program (“863” Program, Nos. 2014AA10A604), the ministry of agriculture key laboratory of the middle and lower reaches of the Yangtze river japonica rice biology and genetic breeding.
Author contributions
W.Y.K. and Z.G.Z. were involved in conceiving the project, designing the experiments and analyzing the data; H.Y.C., Y.J.X. and Y.L. were involving in the map-based cloning of the YGL8 gene; X.W.Y., C.L.W. and Y.Y. were involved in the generation of the transgenic plants; Y.L.W. was involved in YGL8 transient expression in rice protoplasts; W.Y.K. was involved in writing this article; L.L.L. and C.M.W. were involved in revising this article; L.J., H.Q.Z. and J.M.W. were involved in data discussions.
Author information
Authors and Affiliations
Corresponding author
Additional information
Weiyi Kong and Xiaowen Yu have contributed equally to this work.
Electronic supplementary material
Below is the link to the electronic supplementary material.
11103_2016_513_MOESM1_ESM.doc
Supplementary Fig. S1 Phenotypes of the wild-type (WT) and ygl8 mutant plants under different temperature conditions. a Phenotypes of the 2-week old WT and ygl8 mutant seedlings at 12 h light/12 h dark conditions of C20, L30/D20 and C30. Bars: 2 cm. b The Chl content of the 2-week-old WT and ygl8 mutant seedlings. Error bars, ± SD (n = 5).
Supplementary Fig. S2 Transmission electron micrographs of chloroplasts in the upper three leaves from the wild-type (WT) and ygl8 mutant plants. a Chloroplast ultrastructure of the WT plants. g Chloroplast ultrastructure of the ygl8 mutant. d-f and j-l are magnifications of a-c and g-i, respectively. All leaf blades were from the 8-week-old plants. L1, flag leaf; L2, top second leaf; L3, top third leaf. Thy, thylakoid lamellar; SG, starch granule; PG, plastoglobule. Bars: 1 μm (a-c, g-i); 0.5 μm (d-f, j-l). (DOC 9814 KB)
Rights and permissions
About this article
Cite this article
Kong, W., Yu, X., Chen, H. et al. The catalytic subunit of magnesium-protoporphyrin IX monomethyl ester cyclase forms a chloroplast complex to regulate chlorophyll biosynthesis in rice. Plant Mol Biol 92, 177–191 (2016). https://doi.org/10.1007/s11103-016-0513-4
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11103-016-0513-4