Abstract
Norway spruce (Picea abies) defends itself against herbivores and pathogens by formation of traumatic resin ducts filled with terpenoid-based oleoresin. An important group of enzymes in terpenoid biosynthesis are the short-chain isoprenyl diphosphate synthases which produce geranyl diphosphate (C10), farnesyl diphosphate (C15), and geranylgeranyl diphosphate (C20) as precursors of monoterpenes, sesquiterpenes, and diterpene resin acids, respectively. After treatment with methyl jasmonate (MJ) we investigated the expression of all isoprenyl diphosphate synthase genes characterized to date from Norway spruce and correlated this with formation of traumatic resin ducts and terpene accumulation. Formation of traumatic resin ducts correlated with higher amounts of monoterpenes, sesquiterpenes and diterpene resin acids and an upregulation of isoprenyl diphosphate synthase genes producing geranyl diphosphate or geranylgeranyl diphosphate. Among defense hormones, jasmonate and jasmonate-isoleucine conjugate accumulated to higher levels in trees with extensive traumatic resin duct formation, whereas salicylate did not. Jasmonate and ethylene are likely to both be involved in formation of traumatic resin ducts based on elevated transcripts of genes encoding lipoxygenase and 1-aminocyclopropane-1-carboxylic acid oxidase associated with resin duct formation. Other genes involved in defense signalling in other systems, mitogen-activated protein kinase3 and nonexpressor of pathogenesis-related gene1, were also associated with traumatic resin duct formation. These responses were detected not only at the site of MJ treatment, but also systemically up to 60 cm above the site of treatment on the trunk.
Similar content being viewed by others
Introduction
A characteristic feature of conifers in the pine family (Pinaceae) is the production of oleoresin, a very viscous mixture primarily composed of mono-, sesqui-, and diterpenes (Keeling and Bohlmann 2006a, b). Resin-producing structures are present constitutively in many species, but resin accumulation is also induced after herbivore or pathogen attack and probably plays an important defensive role as a physical and chemical barrier against invaders (Franceschi et al. 2005; Krokene et al. 2008a, b). Resin induction involves the differentiation of newly formed traumatic resin ducts (TRD) localized in the young sapwood (Christiansen et al. 1999; Martin et al. 2002), enhanced biosynthesis of terpenoid constituents (Martin et al. 2002; Miller et al. 2005; Zeneli et al. 2006) and induction of genes and enzymes involved in terpenoid biosynthesis (Martin et al. 2002, 2004; Fäldt et al. 2003; Huber et al. 2005; Byun-McKay et al. 2006; Hamberger and Bohlmann 2006; Ro and Bohlmann 2006; Schmidt and Gershenzon 2007, 2008; Schmidt et al. 2010b).
Two main classes of enzymes are involved in the biosynthesis of oleoresin. First, three different kinds of prenyltransferases, referred to collectively as isoprenyl diphosphate synthases (IDS), catalyze the sequential condensation reactions between several units of isopentenyl diphosphate (IPP) and dimethylallyl diphosphate (DMAPP). Reaction products are geranyl diphosphate (GPP, C10), farnesyl diphosphate (FPP, C15), and geranylgeranyl diphosphate (GGPP, C20), the precursors of monoterpenes, sesquiterpenes and diterpenes, respectively. GPP, FPP, and GGPP are each formed by a specific IDS: GPP synthase (GPPS, EC 2.5.1.1), FPP synthase (FPPS, EC 2.5.1.10), and GGPP synthase (GGPPS, EC 2.5.1.30), respectively (Gershenzon and Kreis 1999). Second, terpene synthases (TPS) in combination with cytochrome P450 mono-oxygenases (Cyp P450) convert these linear diphosphates into the huge diversity of terpenes present in conifers (Martin et al. 2004; Keeling and Bohlmann 2006b) (Fig. 1).
Outline of terpenoid oleoresin biosynthesis in conifers showing the condensations of the two C5 diphosphates, isopentenyl diphosphate (IPP) and dimethylallyl diphosphate (DMAPP), to make the larger prenyl diphosphates, geranyl diphosphate (GPP, C10), farnesyl diphosphate (FPP, C15) and geranylgeranyl diphosphate (GGPP, C20). Subsequent cyclizations of these intermediates form the major terpene products in Norway spruce. Thus the isoprenyl diphosphate synthases catalyze the branch-point reactions leading to the different terpene classes
Numerous genes and enzymes involved in induced oleoresin synthesis have been characterized recently from conifers, including some for IDS (Tholl et al. 2001; Burke and Croteau 2002; Schmidt et al. 2005, 2010b; Schmidt and Gershenzon 2007, 2008), some for TPS (Fäldt et al. 2003; Martin et al. 2004; Huber et al. 2005; Byun-McKay et al. 2006; Ro and Bohlmann 2006), and some for Cyp P450 (Hamberger and Bohlmann 2006). Due to their position at a branch point of terpene biosynthesis, IDS are likely to have a key regulatory function in terpene biosynthesis (Martin et al. 2002; Schmidt and Gershenzon 2007, 2008; Schmidt et al. 2010b).
The ecological relevance of terpenoids has been tested in a series of studies using exogenous treatment with methyl jasmonate (MJ) or the free acid jasmonate (JA) to induce conifer defense reactions (Franceschi et al. 2002, Martin et al. 2002, 2003; Fäldt et al. 2003; Hudgins et al. 2003, 2004; Huber et al. 2005; Miller et al. 2005; Ralph et al. 2007). MJ has been shown to increase Norway spruce (Picea abies) resistance to colonization by the spruce bark beetle, Ips typographus, and its virulent fungal associate, Ceratocystis polonica (Erbilgin et al. 2006; Zeneli et al. 2006; Krokene et al. 2008b). Although MJ has been used successfully in several studies to induce and characterize induced defense responses in conifers, endogenous roles of octadecanoids and the putative signal transduction chain for local and systemic responses in conifer defense induction have only been demonstrated in a few studies.
Since formation of TRD results from a transient change of xylem differentiation, other hormones that regulate xylem development, like salicylate (SA) or ethylene, could also have a function in this process. Hudgins and Franceschi (2004) showed that MJ application in Douglas fir (Pseudotsuga menziesii) induced production of ethylene, a jasmonate-linked plant hormone which also acts as an elicitor of conifer defence responses. Ethylene has been shown to increase monoterpene synthesis after wounding and fungal inoculation in Pinus spp. (Peters 1977; Popp et al. 1995). Miller et al. (2005) have demonstrated that feeding by the white pine weevil (Pissodes strobi) results in the induction of genes leading to JA in Sitka spruce (Picea sitchensis) by RNA-blot experiments. Urbanek-Krajnc et al. (2011) showed that application of salicylate induces antioxidant defense responses in the phloem of P. abies and inhibits colonization by I. typographus.
However, there is still limited knowledge about the endogenous levels of hormones in conifers. We also know little about the signal transduction pathways leading to activation and/or de novo formation of resin ducts in local or systemic acquired resistance. More knowledge about the expression patterns of genes involved in octadecanoid and ethylene biosynthesis or other signal cascades could reveal important insights about the regulation of TRD formation and resin biosynthesis.
Here, we demonstrate correlated changes among the formation of TRD, terpene accumulation, and expression of IDS transcripts in Norway spruce. Using the same tissue we are also able to measure endogenous levels of the hormones JA, JA-Ile, and SA and to differentiate between local and systemic induction of genes putatively involved in conifer signalling processes. Our results suggest that IDS catalyze a regulatory step of induced oleoresin production in Norway spruce and that both JA and ethylene-induced signalling pathways are involved in systemic acquired resistance in conifers.
Materials and methods
Chemicals
All chemicals and solvents were analytical grade and were obtained from Merck (http://www.merck.de), Roth (http://www.carlroth.com), Serva (http://www.serva.de), or Sigma (http://www.sigmaaldrich.com).
Accession numbers
Sequence data of MPK3, and NPR1 cDNA-fragments from this article can be found in the NCBI data libraries under accession JF927723 and JF927724. Sequence data of the 13-LOX cDNA-fragment is shown as S1 within the supplemental material.
Plant material
The experimental trees were selected from 12 full-sib Norway spruce (P. abies) families planted out as 1-year-old seedlings at Hogsmark, Ås, SE Norway in 2000. The 12 families originated from controlled crosses between parent trees that were resistant or susceptible to mass-inoculation with Ceratocystis polonica (Brignolas et al. 1998; Christiansen et al. 1999), a virulent blue-staining fungus associated with the spruce bark beetle. We selected three families from crosses between susceptible parents (S × S) with an average of 48.0% blue-stained sapwood after fungal mass-inoculation, and three families from crosses between resistant parents (R × R) with an average of 1.7% blue-stained sapwood (E. Christiansen, unpublished data). Trees from one R × R cross (family 24) and one S × S cross (family 15) were selected for more detailed chemical and molecular analysis, and the parents of these families had an average of 2.0 and 51.0% blue-stained sapwood, respectively.
Ten trees from each of the six full-sib families were treated with an aqueous solution of 50 mM methyl jasmonate (MJ) and 0.1% Tween 20 on the uppermost 6 cm of the 2001 internode on June 22, 2005. On July 14–19 the trees were felled and 10 cm long stem sections were cut at different heights above the treated section (0, 15, 30, 45, and 60 cm above). The upper 2 cm of each section was put on fixative for later microscopy (2% paraformaldehyde and 1.25% glutaraldehyde buffered in 50 mM l-piperazine-N–N′-bis (2-ethane sulfonic) acid, pH 7.2), whereas the remaining 8 cm were frozen in liquid nitrogen. On June 26, 2006 two samples were taken from untreated control trees of family 24 and 15 using the same procedure.
All subsequent chemical and molecular analyses, including measurements of terpene content, hormone quantity and gene transcript level, were carried out on selected frozen samples based on microscopic analyses of traumatic resin duct (TRD) formation. Tree number 7 and 9 of family 24 were selected as examples of MJ-treated trees with extensive formation of TRD, while tree number 3 and 7 of family 15 and tree number 2 of family 24 were investigated as examples of MJ-treated trees with low formation of TRD. Untreated trees from both families were used as controls.
The relative levels of terpenes, hormones and gene transcripts in each sample were obtained by calibration against the mean quantity in MJ-treated tissue in trees with low formation of TRD. Data for control trees and trees with extensive and low formation of TRD are presented as values of individual biological replicates (calculated as the mean of three technical replicates per biological replicate). Combined data for all sampling distances away from the MJ treatment site are presented as boxplots in the supplemental material (Figs. S1–S4).
Microscopy preparation and image analysis
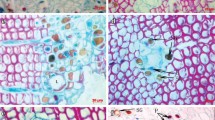
Anatomical samples from all trees were processed for light microscopy as described by Franceschi et al. (2000). Briefly, samples were rinsed with buffer, dehydrated in ethanol and embedded in acrylic resin. Cross sections (1 μm thick) were cut with an ultra-microtome, dried onto gelatin-coated slides, stained with Stevenel’s blue, and mounted with immersion oil. Digital images were taken with a Leitz Aristoplan photo-microscope fitted with a Leica DC300 CCD camera (Leica, Wetzlar, Germany), using bright field optics.
To quantify the extent of TRD in the sapwood of all investigated samples, cross-sections were imaged at 16× magnification, and the percent coverage of resin ducts (including the epithelial cells lining the ducts) was measured across 870 μm in the tangential direction. Percentage data were arcsine transformed and subjected to analysis of variance using JMP (SAS Institute, NC). Data are presented as means + 95% confidence intervals, n = 26–30 for resistant trees and 25–29 for susceptible trees for the different sampling positions along the stem.
Extraction of resin terpenes
Terpene extractions of bark and sapwood samples were based on the procedures of Martin et al. (2002). All steps of the sample preparation were carried out in 2 ml vials with a Teflon-coated screw cap (Hewlett-Packard, Palo Alto, CA, USA). Sample pieces (200 mg) were submerged into 1.5 ml of tert-butyl methyl ether containing 150 μg/ml isobutylbenzene and 200 μg/ml pimaric acid as internal standards, and extracted for 14 h with constant shaking at room temperature.
To purify extracted terpenes from other organic acids, the ethereal extract was transferred to a fresh vial and washed with 0.3 ml of 0.1 M (NH4)2CO3 (pH 8.0). The extract was then split into two equal portions. For the analysis of diterpene resin acids, one aliquot was methylated by adding 50 μl of 0.2 M N-trimethylsulfonium hydroxide in methanol (Macherey-Nagel, Düren, Germany) to 0.4 ml of the washed ethereal extract in a separate vial. After incubation at room temperature for 2 h, the solvent was evaporated under nitrogen to leave 100 μl of sample, which was stored at −20°C until analysis by GC-MS. For the analysis of monoterpenes and sesquiterpenes, the remainder of the original sample was prepared for GC-MS analysis by filtering through a Pasteur pipette column filled with 0.3 g of silica gel (Sigma 60 Å) overlaid with 0.2 g of anhydrous MgSO4. The column was washed with 1 ml of diethyl ether, and the combined eluant collected in a new vial and evaporated to an approximate volume of 100 μl.
Analysis of monoterpenes, sesquiterpenes and diterpenes
GC-MS analysis of monoterpenes and sesquiterpenes was carried out with a Hewlett-Packard 6890 system, using a DB-WAX column [0.25 mm × 0.25 μm × 30 m (J&W Scientific, Folsom, CA, USA)]. Split injections (1 μl ethereal extract) were made at a ratio of 1:5 with an injector temperature of 220°C. The instrument was programmed to hold an initial temperature of 40°C for 3 min and then increased at a rate of 3°C min−1 to 80°C. The temperature was then increased at a rate of 5°C min−1 to 180°C with a 5 min hold, followed by a final ramp of 15°C min−1 up to 240°C. Helium was used as carrier gas at a constant flow of 1 ml min−1. The MS detector was operated using the total ion mode at a temperature of 230°C. For the identification of compounds, the MS detector was operated using a mass range of 40–350 and spectra were collected under standard conditions (electron impact ionization at 70 eV).
Analysis of diterpene constituents was performed on the same GC-MS instrument fitted with an HP-5 column (0.25 mm × 0.25 μm × 30 m, Hewlett-Packard). Injections were made with 1 μl of the concentrated, derivatized ethereal extract. GC-MS split ratios were 1:10 with an injector temperature of 220°C. The instrument was programmed for an initial temperature of 120°C and increased at a rate of 1°C min−1 to 150°C, followed by 5°C min−1 up to 280°C (6 min hold). Helium was used as carrier gas at a constant flow of 1 ml min−1. For identification of compounds the MS detector was operated using a mass range of 40–550.
GC-MS generated peaks were quantified using Hewlett-Packard Chemstation software. Isobutylbenzene was used as the internal standard for quantification of monoterpenes and sesquiterpenes, while methylated pimaric acid was used as an internal standard to calculate diterpene concentrations. For quantitative analysis of monoterpenes and sesquiterpenes, the MS detector was operated in the single ion mode monitoring ions at m/z 91, 93 and 161. For diterpene quantification ions were monitored at m/z 121, 135, 239 and 241. Identification of terpenes was based on comparison of retention times and mass spectra with authentic standards (Sigma) or mass spectra in the Wiley or National Institute of Standards and Technology libraries.
Analysis of jasmonic acid, jasmonate-isoleucine conjugate and salicylate
The analysis of jasmonic acid (JA), jasmonate-isoleucine conjugate (JA-Ile), and salicylate (SA) were based on the procedures of Schmidt et al. (2010a). Approximately 0.1 g of ground sapwood was homogenized in 1 ml methanol spiked with 40 ng of [2H2]JA, 40 ng [2H4]SA, and 8 ng of [13C6]JA-Ile by shaking for 30 min. Homogenates were centrifuged at 20,000g for 20 min at 4°C, the methanol phase collected, and the homogenate re-extracted with 1.0 ml methanol. The organic phases were combined and the samples evaporated to dryness in a vacuum concentrator at 30°C. The dry residue was reconstituted in 0.5 ml of 70% (v/v) methanol/water and analyzed by an API 3200™ liquid chromatography–triple quadrupole mass spectrometry system (Applied Biosystems, Darmstadt, Germany) coupled to a Agilent 1200 HPLC system (Agilent Technologies, Böblingen, Germany). 10 μl samples were injected onto a Zorbax Exclipse XDB-C18 column (50 × 4.6 mm, 1.8 μm diameter, Agilent Technologies). The mobile phase, comprised of solvent A (0.05% formic acid) and solvent B (acetonitrile), was used in a gradient of (min/%B): 0/5, 0.5′/5, 4.5′/90, 4.52′/100, 5′/100, 5.1′/5, 8.5′/5) with a flow rate of 0.8 ml min−1. Compounds were detected as negative ions in a multiple reaction monitoring mode. Molecular ions [M–H]− at m/z 136.8, 209.0 and 322.1 generated from endogenous SA, JA, and JA-Ile and from their internal standards 140.8, 213.0 and 328.1 were fragmented under −22, −28 and −32 V CE for SA, JA, and JA-Ile, respectively. The product ions are m/z 93.1 and 97.1 for SA and its internal standard respectively, m/z 59.1 and 61.1 for JA and its internal standard, and m/z 130.1 and m/z 136.1 for JA-Ile and its internal standard. The ratio of ion intensities of the product ions was used to quantify SA, JA and JA-Ile.
Identification of genes involved in the signal transduction chain of local or systemic acquired resistance in Norway spruce
To investigate the possible roles of genes involved in the signal transduction chain of local or systemic acquired resistance in Norway spruce, cDNA sequences with similarities to those of known candidate genes from other species were identified by using an EST database developed by the NSF Genomics of Loblolly Pine Embryogenesis Project (http://compbio.dfci.harvard.edu). The search revealed contig TC68325 as a putative partial sequence of a lipoxygenase (13-LOX). Putative sequences of non-expressor of pathogenesis-related protein 1 (NPR1) and mitogen-activated protein kinase 3 (MPK3) from Sitka spruce were identified by using the database and clone bank TREENOMIX of the Conifer Forest Health EST sequencing project (http://www.treenomix.ca; Ralph et al. 2008).
cDNA fragments corresponding to these sequences were isolated and cloned using PCR with appropriate primers and cDNA of MJ-treated sapwood tissue of replicate 9 (family 24) as template. Comparison of the deduced amino acid sequences to the NCBI-database (http://www.ncbi.nlm.nih.gov) revealed significant similarities to characterized plant sequences. The 13-LOX homologue fragment contained 175 bp and showed 70% amino acid identity to a 13-LOX (CAA65269.1) cloned and characterized from Solanum tuberosum (Royo et al. 1996). For the NPR1 fragment, the deduced amino acid sequence of the 309 bp cDNA fragment showed 72% amino acid identity to a NPR1 gene from Capsicum annuum (DQ648785.1), whereas sequence alignment of the 366 bp putative MPK3 fragment revealed 80% amino acid identity to the MPK3 gene of Gossypium barbadense (AF531365.1).
Quantitative real-time PCR
Total RNA from bark or sapwood was isolated using the Plant RNA Isolation Kit (Invitek, Berlin, Germany) according to the manufacturer’s instructions, but including an additional DNA digestion step [RNase Free DNase Set (Qiagen, Hilden, Germany)]. Using identical amounts of total RNA, template cDNA for subsequent PCR reactions was generated using Superscript™ III (Invitrogen, Carlsbad, CA, USA) according to the manufacturer’s instructions. Quantitative PCR was performed with Brillant® SYBR Green QPCR Master Mix (Agilent Stratagene, Santa Clara, CA, USA). Quantitative real-time PCR primers for PaIDS1-6 were designed using specific criteria, including predicted melting temperature of at least 58°C, primer length of 22–24 nucleotides, guanosine-cytosine content of at least 48% and an amplicon length of 120–150 bp. Primer sequences are listed in Schmidt and Gershenzon (2007, 2008) and Schmidt et al. (2010b). Primers used for quantative amplification of MPK3, NPR1, 13-LOX or ACO are shown in Table S2 (see supplemental material). For MPK3, NPR1, and 13-LOX specific Norway spruce sequences were obtained from isolated and cloned cDNA fragments, whereas sequences for ACO came from the NCBI database (http://www.ncbi.nlm.nih.gov) under the accession number DQ480740 (Hudgins et al. 2006).
Primer specificity was confirmed by melting curve analysis, by an efficiency of product amplification of 1.0 ± 0.1, and by sequence verification of at least eight cloned PCR amplicons for each PCR reaction per gene. Reactions with water instead of cDNA template were run with each primer pair as a control. A standard thermal profile of 95°C for 10 min, then 45 cycles of 95°C for 30 s, 53°C for 30 s and 72°C for 30 s was used. The fluorescence signal was captured of the end of each cycle, and a melting curve analysis was performed from 53 to 95°C with data capture every 0.2°C during a 1 s-hold. Quantitative real time PCR reactions were performed as described in the operator’s manual using a Stratagene MX3000P™. Transcript quantity is the average of three determinations each of three replicate samples from 2 to 3 biological replicates. All amplification plots were analyzed with the MX3000P™ software to obtain threshold cycle (Ct) values. Transcript abundance was normalized with the transcript abundance of the ubiquitin gene (Schmidt and Gershenzon 2007).
Results
Traumatic resin ducts were more abundant in offspring from parents resistant to a bark beetle-associated fungus than in offspring from susceptible parents
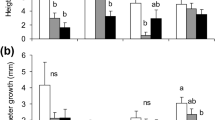
Most methyl jasmonate (MJ) treated Norway spruce (P. abies) trees in this study exhibited traumatic resin ducts (TRD) in the newly formed xylem (Fig. 2), but trees originating from crosses between parents resistant to the bark beetle-associated fungus, Ceratocystis polonica, showed much stronger morphological changes in response to treatment than trees from crosses between susceptible parents. MJ treatment induced significantly more TRD in offspring from resistant parents than in offspring from susceptible parents (F 1,56 = 7.36, P = 0.009; average for all sampling positions per tree) (Fig. 3). The increase in TRD was found both at the site of MJ treatment (F 1,57 = 6.60, P = 0.01) and at sampling positions 15-60 cm above the MJ treatment site (F 1,56 = 3.62 P = 0.06; all distant sampling positions combined).
Cross sections from the methyl jasmonate (MJ) treated sections of young Norway spruce stems about 25 days after MJ treatment. a Tree 24/2 showed no formation of traumatic resin ducts at any sampling position. b Tree 24/9 displayed extensive formation of TRD covering up to 71% of the sapwood circumference in the measured cross-sections. Abbreviations: C cambium, PP polyphenolic parenchyma cells, R radial ray, S sieve cells, X xylem, TRD traumatic resin duct
Amount of traumatic resin ducts in the sapwood of Norway spruce offspring from parents resistant (black bars) or susceptible (white bars) to the bark beetle-associated fungus, Ceratocystis polonica, after partial treatment of the stem with methyl jasmonate. Resin ducts were quantified on sapwood samples collected at various distances above the treatment site ~4 weeks after treatment. Duct area was significantly greater in offspring from resistant versus susceptible parents (ANOVA for all sampling positions combined: F 1,56 = 7.36, P = 0.009). Data are presented as mean + 95% confidence interval for 26–30 resistant trees and 25–29 susceptible trees per sampling position
There was considerable variation in TRD within families, ranging from 0 to >60% coverage at a given sampling position in both resistant and susceptible families. For this reason, the analyses in the remainder of this study were performed on a subset of trees from families 15 and 24 with contrasting expression of TRD. Trees with extensive formation of TRD after MJ treatment (70% of sapwood circumference at treatment site, 35–53% of circumference up to 45–60 cm away from treatment site) were designated “high TRD”, while trees which showed low formation of TRD after MJ treatment (<20% of sapwood circumference, only at treatment site) were designated “low TRD” (Table S3, supplemental material). Unsprayed control trees of the same families were also analyzed.
Terpene accumulation was highest in trees with more traumatic resin ducts
To correlate terpene levels in Norway spruce with formation of TRD, we measured terpene concentrations in the bark and sapwood of high and low TRD trees following MJ treatment. High TRD trees had much higher terpene levels in the sapwood than low TRD trees or unsprayed controls, but no differences between trees were found in the bark (data not shown).
Total monoterpenes were up to 4.4-fold more abundant in high TRD trees compared to low TRD trees and untreated controls, and showed a different concentration pattern along the stem (Fig. 4; Table 1). The highest monoterpene content was detected at the treatment site itself (1,080 μg × g dry weight−1) and 15 cm above the treatment site (1,485 μg × g dry weight−1), with a gradual decrease further up the stem towards the level found in control trees. In low TRD trees or in untreated controls, no differences in monoterpene content were observed at any sampling position along the stem. The main monoterpenes detected in the sapwood were α-pinene and ß-pinene (571 and 387 μg × g dry weight−1, respectively, at the treatment site) in addition to camphene, sabinene, δ-3-carene, myrcene, limonene, and ß-phellandrene (Table 1). For all sampling positions combined high TRD trees had much higher total monoterpene levels than low TRD trees and controls (median 2.6 vs. 0.7; Fig. S1).
Relative amounts of major monoterpenes (MT), sesquiterpenes (ST), and diterpenes (DT) in Norway spruce sapwood after methyl jasmonate (MJ) treatment in high and low traumatic resin duct (TRD) trees and in unsprayed control trees (Ctr.). Sapwood was collected at various distances above the treatment site ~4 weeks after treatment. The mean terpene content at the MJ treatment site in low TRD trees was set to 1.0. Data are presented as values of 2–3 biological replicates (each calculated as the mean of three technical replicates). The absolute levels corresponding to the 1.0 value are 246 μg × g dry wt−1 for monoterpenes, 0.37 μg × g dry wt−1 for sesquiterpenes, and 243 μg × g dry wt−1 for diterpenes
Total sesquiterpene content was up to 13.6-fold higher in the trees with high TRD formation compared to low TRD trees and untreated controls. The concentration pattern along the stem was comparable to that found for monoterpenes. The highest sesquiterpene content was detected at the treatment site itself (5.0 μg × g dry weight−1) and 15 cm above the treatment site (6.3 μg × g dry weight−1) (Fig. 4, Table 1). However, total sesquiterpene concentration was only about 5% of that of mono- or diterpenes. The main sesquiterpenes detected in sapwood were (E)-β-caryophyllene and δ-cadinene (1.42 and 1.25 μg × g dry weight−1, respectively, at the treatment site). Other sesquiterpenes detected were (E)-β-farnesene, germacrene D, and longipinene (Table 1). For all sampling positions combined high TRD trees had much higher total sesquiterpene levels than low TRD trees and controls (median 5.9 vs. 1.4 and 0.7, respectively; Fig. S1).
Total diterpene content was up to sevenfold higher in the trees with high TRD formation (1,700 μg × g dry weight−1) than in the other trees. The highest diterpene content (943 μg × g dry weight−1 for levopimarate) was detected at the MJ treatment site (Fig. 4; Table 1). Diterpenes in the sapwood included levopimarate, abietate, neoabietate, isopimarate, dehydroabietate, and sandarocopimarate. For all sampling positions combined high TRD trees had higher total diterpene levels than low TRD trees and controls (median 2.5 vs. 1.0 and 0.5, respectively; Fig. S1).
Transcripts of isoprenyl diphosphate synthases were higher in stem tissues with more traumatic resin ducts
Expression of isoprenyl diphosphate synthase (IDS) genes was investigated to determine if transcript abundance was correlated with the formation of TRD. Transcript levels of six different IDS genes were measured by quantitative real-time PCR in the same trees used for terpene analyses (Fig. 5).
Relative abundance of mRNA transcripts of PaIDS1, PaIDS2, PaIDS5, and PaIDS6 genes in Norway spruce sapwood (a) and bark (b) after methyl jasmonate (MJ) treatment in high and low traumatic resin duct (TRD) trees and in unsprayed control trees (Ctr.). Samples were collected at various distances above the MJ treatment site 4 weeks after treatment. Transcript abundance of each IDS gene was measured by quantitative real-time PCR using SYBR-Green for detection and normalized against ubiquitin. The mean transcript abundance at the MJ treatment site in low TRD trees was set to 1.0. Data are presented as values of 2–3 biological replicates (each calculated as the mean of three technical replicates)
In the sapwood of trees with a high degree of TRD formation after MJ treatment, enhanced transcript levels were detected for PaIDS1, PaIDS2, and PaIDS5. At the site of MJ treatment, transcript abundance was up to 12-fold greater for PaIDS1, 2.2-fold for PaIDS2 and 22-fold for PaIDS5 relative to the expression level detected in trees with low formation of TRD after MJ treatment. Fifteen centimeters above the MJ treated stem section, expression remained high, but sixty centimeters away expression decreased to sixfold, onefold and fourfold for PaIDS1, PaIDS2, and PaIDS5, respectively. In the trees with low formation of TRD, expression levels of PaIDS1, PaIDS2 and PaIDS5 were slightly higher than the constitutive levels detected in the untreated control trees. For PaIDS6, no changes in expression levels were measured in the sapwood, neither in trees with high nor low formation of TRD (0.5-fold to 1.0-fold changes relative to the mean expression level in trees with low formation of TRD) (Fig. 5a). For all sampling positions combined high TRD trees had much higher expression levels for PaIDS1, PaIDS2, and PaIDS5 in the sapwood than low TRD trees and controls (median 8.4, 1.6 and 7.4 vs. 0.1–0.9; Fig. S2a).
For PaIDS3 and PaIDS4, no changes in expression levels were measured in the sapwood (data not shown).
In the bark of trees with high formation of TRD, MJ treatment induced enhanced transcript levels mainly of PaIDS1 and PaIDS5. The highest transcript abundances detected were 5.8-fold and 10-fold increases, respectively at the treatment site relative to the expression level at the treatment site in the bark of low TRD trees. Further up the stem, relative transcript abundance decreased almost to the constitutive expression levels found in the controls. Transcript levels of PaIDS6 in the bark of trees with high formation of TRD increased and then decreased along the stem moving away from the treatment site, with the highest relative abundance (2.4-fold increase) 30 cm above the treatment site. For PaIDS2 and for PaIDS3 and PaIDS4 (data not shown), no clear changes in expression levels were detected in the bark, neither between trees nor sampling positions within trees (0.5-fold to 1.4-fold changes relative to the mean expression level in trees with low formation of TRD) (Fig. 5b). For all sampling positions combined high TRD trees had higher expression levels for PaIDS1, PaIDS2, and PaIDS5 in the bark than low TRD trees and controls (median 2.5, 2.4 and 1.7 vs. 0.3–0.9; Fig. S2b).
Accumulation of jasmonate and jasmonate-isoleucine conjugates, but not salicylate, was associated with formation of traumatic resin ducts
To compare resin duct formation and endogenous levels of possible defense signaling hormones in Norway spruce, we measured jasmonate (JA), jasmonate-isoleucine conjugate (JA-Ile), and salicylate (SA) concentrations in sapwood and bark from trees with high and low levels of TRD after MJ treatment. In general, absolute JA and JA-Ile levels were much higher in the bark (2.9 μg and 28.0 ng × g dry weight−1, respectively) than in the sapwood (29.0 ng JA and 3.5 ng JA-Ile × g dry weight−1). Trees with extensive formation of TRD had much higher levels of JA and JA-Ile compared to trees with a low amount of TRD or unsprayed controls, both in the sapwood and in the bark. SA levels were higher at the treatment site in high TRD trees compared to low TRD trees and control trees, but only for the bark and not the sapwood. No clear absolute differences in SA were observed in the sapwood or bark for any other trees or treatments.
In the sapwood, JA and JA-Ile had a similar pattern of concentration along the stem, peaking 15 cm above the treatment site in high TRD trees at levels that were 6-fold and 12-fold higher, respectively than in low TRD trees and control trees (Fig. 6). For all sampling positions combined high TRD trees had much higher levels of JA and JA-Ile in the sapwood than low TRD trees and controls (median 4.2 and 5.0 vs. 0.1-0.8; Fig. S3). No changes were found for SA in the sapwood.
In the bark, relative JA levels were about twofold higher in high TRD trees than in low TRD trees. JA-Ile levels were up to 4.5-fold higher in trees with high formation of TRD than in other trees. The peak in JA-Ile was detected 15 cm above the treatment site. SA levels in the bark were around 2.3-fold higher in trees with high formation of TRD compared to low TRD and control trees (50.6 and 22.0 ng × g dry weight−1, respectively) (Fig. 6). For all sampling positions combined high TRD trees had much higher levels of JA-Ile (but not JA) in the bark than low TRD trees and controls (median for JA-Ile = 2.6 vs. 0.2 and 0.05 for low TRD and controls, respectively; Fig. S3).
Relative amounts of jasmonate (JA), jasmonate-isoleucine conjugate (JA-Ile) and salicylate (SA) in Norway spruce sapwood (black circles) and bark (white circles) after methyl jasmonate (MJ) treatment in high and low traumatic resin duct (TRD) trees and in unsprayed control trees (Ctr.). The mean hormone content at the MJ treatment site in low TRD trees was set to 1.0. Data are presented as values of 2–3 biological replicates (each calculated as the mean of three technical replicates). The absolute levels corresponding to the 1.0 value in sapwood or bark are 4.8 ng or 1.4 μg × g dry wt−1 for jasmonate, 0.3 ng or 6.2 μg × g dry wt−1 for the jasmonate-isoleucine conjugate, and 15 or 22 ng × g dry wt−1 for salicylate
Genes involved in JA, SA and ethylene defense signaling were induced in trees with abundant traumatic resin ducts
The relative expression levels of four different genes thought to be involved in defense signal transduction in MJ-treated Norway spruce stems were investigated by quantitative real-time PCR. Increased transcript abundance was detected in the sapwood of trees with high formation of TRD, compared with low TRD trees and unsprayed control trees, whereas transcript levels in the bark differed only slightly among trees (Fig. 7). In sapwood, relative expression levels increased around fourfold for non-expressor of pathogenesis-related protein 1 (NPR1), a positive regulator of systemic acquired resistance against pathogens (Dong 2004), and 20-fold for 1-aminocyclopropane-1-carboxylate oxidase (ACO), encoding the final step in ethylene biosynthesis (Wang et al. 2002), at the MJ-treated stem section or 15 cm above it. Expression increased in the same pattern sixfold for 13-lipoxygenase (13-LOX) encoding an early step in JA biosynthesis (Wasternack and Kombrink 2010), and fourfold for mitogen-activated protein kinase 3 (MPK3), also part of JA signaling transduction (Rodriguez et al. 2010). Further up the stem away from the treatment site, transcript levels in the sapwood decreased for all investigated genes. In the bark, expression levels of all genes increased somewhat (up to threefold) in trees with high formation of TRD (Fig. 7). No clear changes in expression were detected in the sapwood or bark of trees with low formation of TRD or in untreated controls. For all sampling positions combined high TRD trees had higher expression levels for NPR1, MPK3, 13-LOX, and ACO in the sapwood than low TRD trees and controls (median 2.0, 3.4, 3.8 and 2.5 vs. 1.0–1.2; Fig. S4). In the bark median expression levels were lower and differed less between trees (around 2.0 in high TRD trees vs. 1.1 in the controls (Fig. S4).
Relative abundance of mRNA transcripts of NPR1, MPK3, 13-LOX, and ACO genes in Norway spruce sapwood (black circles) and bark (white circles) after methyl jasmonate (MJ) treatment in high and low traumatic resin duct (TRD) trees and in unsprayed control trees (Ctr.). Transcript abundance of each gene was measured by quantitative real-time PCR using SYBR-Green for detection and normalized against ubiquitin. The mean transcript abundance at the MJ treatment site in low TRD trees was set to 1.0. Data are presented as values of 2–3 biological replicates (each calculated as the mean of three technical replicates)
Discussion
Traumatic resin duct formation is correlated with oleoresin accumulation in the sapwood of Norway spruce
Traumatic resin ducts (TRD) filled with oleoresin appear to be an important defensive feature against insects and pathogens in many conifers in the pine family (Huber et al. 2004; Heijari et al. 2005; Franceschi et al. 2005), including e.g. white spruce (Picea glauca), Sitka spruce (Picea sitchensis), and Norway spruce (Alfaro 1995; Tomlin et al. 1998; Nagy et al. 2000). TRD add considerably to a tree’s overall oleoresin reservoir since they are much larger and more abundant than ordinary axial resin ducts in the sapwood, with a volume that is about four times greater than axial ducts per unit length in Norway spruce (Krokene et al. 2008b). After methyl jasmonate (MJ) treatment, the pattern of terpene accumulation in sapwood along the stem (Fig. 4) corresponded closely to the pattern of TRD formation (Fig. 3), suggesting that the TRDs were indeed the major repository of terpenes in the sapwood.
The oleoresin within Norway spruce TRD in this study was found to be composed primarily of mono- and diterpenes, with smaller amounts of sesquiterpenes, and had a composition similar to that reported in previous studies (Martin et al. 2002; Zeneli et al. 2006). Whereas mono- and diterpene levels in the sapwood increased fourfold to sevenfold after MJ treatment in trees with abundant TRD (relative to levels in trees with low TRD), sesquiterpene levels were up to 17 times higher in trees with high TRD. Traumatic resin ducts were also induced in the stem up to 60 cm away from the site of MJ treatment with the same shift towards sesquiterpene production. Some of this increase may consist of volatiles that are later emitted from the stems and not stored inside TRD. Martin et al. (2003) reported that the emission of certain sesquiterpenes increased up to 100-fold in young Norway spruce plants after MJ treatment. Whatever the reason, the different ratios of terpene types found when comparing oleoresin at the MJ treatment site to that 60 cm away suggest that resin in TRDs has a terpene composition distinct from that in constitutive resin ducts.
Transcription of GPP and GGPP synthase genes is closely correlated with traumatic resin duct formation
We investigated the expression of six isoprenyl diphosphate synthase (IDS) genes that catalyze branch-point reactions leading to the different classes of terpenes in Norway spruce (Table 2) (Schmidt and Gershenzon 2007, 2008; Schmidt et al. 2010b). Of the six, the induction pattern of three genes, PaIDS1, PaIDS2, and PaIDS5, within the stem sapwood correlated closely with formation of TRD, suggesting that these genes and the enzymes they encode are localized in the sapwood (Figs. 3, 5a). IDS2 produces geranyl diphosphate (GPP), the substrate for the monoterpenes; IDS5 produces geranylgeranyl diphosphate (GGPP), the substrate for the diterpenes; while IDS1 produces mostly GPP with some GGPP (Table 2). Within the sapwood, the transcripts most likely reside in the epithelial cells of the TRD, as was demonstrated recently for PaIDS1 (Schmidt et al. 2010b). In the bark, IDS gene induction (Fig. 5b) is probably involved in terpene biosynthesis in other cell or tissue types, such as the radial resin ducts inside the radial rays and the large cortical resin ducts (Krokene et al. 2008b; Abbott et al. 2010).
The induction of PaIDS1, PaIDS2, and PaIDS5 at the site of MJ treatment confirms the central role of these three IDS genes in induced conifer defense reactions (Schmidt and Gershenzon 2007, 2008; Schmidt et al. 2010b). The maximum induction found for PaIDS1 in the sapwood by MJ relative to untreated sapwood was comparable to that previously found in MJ treated saplings (Schmidt et al. 2010b). In contrast, PaIDS2 induction by MJ was only one third of that found previously, whereas PaIDS5 induction by MJ was four times higher (Schmidt and Gershenzon 2007, 2008). These differences may be due to genetic variation between the spruce genotypes used or changes in susceptibility to MJ at different stages of development. In systemic induced sapwood tissue, highly elevated transcript levels for PaIDS1, PaIDS2, and PaIDS5 were found 15 cm above the MJ-treated stem section, and transcript levels remained higher than in the unsprayed controls even further up the stem.
In bark tissues after MJ spraying, PaIDS1, PaIDS5, and to a lesser degree PaIDS6, also showed increased transcript levels at the site of treatment. However, maximum induction in the bark was only 50% of that in the sapwood, and well below the levels of PaIDS1 and PaIDS5 induction (relative to untreated bark) recently found in MJ-treated saplings (Schmidt and Gershenzon 2007; Schmidt et al. 2010b). Some of the IDS activity in the bark may be an artifact if parts of the cambium adhered to the bark during sampling, since TRD may begin to differentiate while the developing cells are still in the cambial region.
Away from the site of MJ treatment, 30–60 cm further up the trunk, slightly increased IDS transcript levels were detected in the bark (relative to levels in unsprayed bark tissue), but these differences were much less than those found in the sapwood. PaIDS6 seemed to follow a different pattern than PaIDS1 and PaIDS5 with no or only very modest induction locally at the MJ-treated site, but slightly higher transcript levels further up the stem. However, compared with the main IDS genes involved in induced conifer defense (PaIDS1, PaIDS2, and PaIDS5) transcript levels of PaIDS6 remained low, supporting a smaller role in induced defense responses (Schmidt and Gershenzon 2007).
The overall increase in mono- and diterpene concentrations in the sapwood correlates well with the induction of the IDS genes that encode the enzymes making their precursors- PaIDS1 (GPP and GGPP), PaIDS2 (GPP) and PaIDS5 (GGPP). However, the observed increase in sesquiterpene levels in the sapwood is not matched by an increase in the corresponding IDS gene encoding FPP synthesis, PaIDS4 (Schmidt and Gershenzon 2007). Because the other Norway spruce IDS monitored in this study either have no detectable FPP synthase activity (IDS1, 2 and 5, Schmidt and Gershenzon 2007, 2008; Schmidt et al. 2010b), or did not show an increase in transcript (PaIDS3), an additional FPP synthase, a bifunctional GPP/FPP synthase or a GGPP synthase also releasing FPP may be present in the sapwood after induction. Regarding PaIDS3, it was recently shown in Arabidopsis thaliana that a gene with close sequence similarity to PaIDS3 encodes an enzyme synthesizing C25–C40 long isoprenyl diphosphate products with double bonds in an (E)-orientation (Hsieh et al. 2011). Thus involvement of IDS3 in induced oleoresin biosynthesis seems unlikely, as already suggested (Schmidt and Gershenzon 2008).
Jasmonate, but not salicylate signaling is involved in traumatic resin duct formation
To understand how traumatic resin duct formation is triggered, how the various aspects of the defense response (e.g. formation of TRD, terpene production) are integrated, and how a defense signal is propagated from a single site to other locations on the tree, it is necessary to identify the hormones and signaling mechanisms involved. Recent reviews suggest that jasmonic acid (JA) and ethylene are involved in defense signal transduction in conifers (Phillips et al. 2006; Zulak and Bohlmann 2010). The large number of studies that have induced conifer defence responses with exogenous MJ application (Franceschi et al. 2002; Martin et al. 2002; Hudgins et al. 2003, 2004; Huber et al. 2005; Zhao et al. 2010; Zulak et al. 2009, 2010; Hall et al. 2011) are the principal evidence for an essential role of endogenous jasmonates. However, JA has never been previously measured in intact conifers, only in cell cultures (Mueller et al. 1993; Phillips et al. 2007), and its bioactive form, the jasmonate-isoleucine conjugate (JA-Ile, Fonseca et al. 2009), has not been reported from conifers of any form. Here we detected both JA and JA-Ile in all of our samples, but in much higher levels associated with high TRD formation and greater accumulation of mono- and sesquiterpenes. All previous studies of MJ application in conifers have focused on local tissues, whereas here we also investigated systemic responses in stem parts above the treatment site. JA and JA-Ile accumulation in sapwood of high TRD trees actually increased 15 cm above the local treatment site and then declined to levels below treatment at 45 cm. This correlated very well with the pattern found for monoterpene and sesquiterpene accumulation, suggesting that JA and JA-Ile cascades are involved in triggering terpenoid biosynthesis. In bark, elevated JA levels were found only at the treatment site, possibly a residue of applied MJ that has undergone hydrolysis or an artifact caused by cambium adhering to the bark during sampling. However, the fact that we found much higher JA and JA-Ile levels in high than in low TRD trees and a much stronger response in sapwood than in bark (even though MJ was applied to the bark) clearly suggests that the JA and JA-Ile we measured are not just metabolites of the applied MJ, but reflect endogenous changes associated with defense induction.
There is considerable knowledge about the genes involved in JA biosynthesis in conifers from the sequencing of more than 200,000 ESTs from white spruce (Picea glauca) and Sitka spruce (Picea sitchenis) in the TREENOMICS database (Ralph et al. 2008), and from DNA microarray experiments demonstrating gene induction following mechanical wounding or feeding by the spruce budworm (Choristoneura occidentalis) or the white pine weevil (Pissodes strobi) (Ralph et al. 2006). 13-lipoxygenase (13-LOX) is a key enzyme leading to JA formation, catalyzing the conversion of α-linolenic acid to 13(S)-hydroxyperoxylinolenic acid (Royo et al. 1996; Wasternack 2007). Another segment of the JA signal transduction cascade prior to 13-LOX involves mitogen-activated protein kinases (MPK), with MPK3 being a key gene in JA signalling in Arabidopsis (Takahashi et al. 2007). In high vs. low TRD trees and in sapwood vs. bark, higher endogenous JA levels correlated with the induction of 13-LOX and MPK3, suggesting a direct relationship between transcriptional activation of these two genes and JA and JA-Ile based defense signaling in P. abies.
In contrast to JA and JA-Ile, we found little evidence for involvement of salicylate (SA) in systemic formation of TRD in our spruce trees, neither in bark nor in wood. However, greater accumulation of SA was found in the bark at the MJ treatment site in high TRD vs. low TRD or unsprayed control trees, suggesting a possible role for SA in TRD induction. In previous studies, increases in bound and free SA have been detected in roots of several-week-old Norway spruce seedlings inoculated with Pythium sp. or treated with MJ (Kozlowski and Metraux 1998; Kozlowski et al. 1999). To support the involvement of SA, expression data from genes involved in SA signalling would be valuable. Because we were unable to identify a gene encoding isochorismate synthase, the key step of SA biosynthesis, among available P. abies sequences (due to the lack of sequence similarity to angiosperm genes), we used NPR1 as an indicator of SA signaling. This gene encodes a central positive regulator of systemic acquired resistance signaling in many angiosperms, whose level is modulated by cross-talk between SA- and JA-dependent defence pathways (Spoel et al. 2003; Fobert and Despres 2005; Dong 2004). We detected higher induction of NPR1 in high vs. low TRD trees and in the sapwood vs. the bark. This pattern correlates well with those for 13-LOX and MPK3, suggesting possible participation of SA-dependent signalling in spruce TRD formation.
In addition to the hormones JA, JA-Ile, and SA, ethylene has also been reported to be involved in induction of conifer defense. Ethylene induces the same anatomical and cellular defence responses as insect feeding, mechanical wounding, or fungal inoculation in Douglas fir (Pseudotsuga menziesii) (Hudgins and Franceschi 2004). The final step in ethylene biosynthesis is catalyzed by 1-aminocyclopropane-1-carboxylate oxidase (ACO) and the encoding gene has been used as a marker gene for involvement of ethylene in signal transduction. Although we did not quantify ethylene accumulation, much higher transcript levels of ACO at the MJ treatment site in the sapwood of high TRD vs. low TRD and unsprayed control trees hint that ethylene biosynthesis may have been upregulated in high TRD trees. In addition, because of the reported link between mitogen-activated protein kinase cascades and ethylene signalling (Ouaked et al. 2003), increased expression of MPK3 also suggests that ethylene signalling may be involved in the formation of TRD.
Despite the importance of induced terpenoid biosynthesis in conifer defense, there are still many unsolved questions about its regulation. In the present manuscript we have provided new information about the involvement of IDS expression in formation of TRD and the roles of various hormones in the signalling process. Further work is needed to clarify the relative importance of JA, SA and ethylene and the participation of other components in signalling cascades. Greater knowledge of the regulation of terpenoid induction in P. abies should contribute to a deeper understanding of conifer defence and new approaches to manage many of the destructive pests of conifer forests.
References
Abbott E, Hall D, Hamberger B, Bohlmann J (2010) Laser microdissection of conifer stem tissues: isolation and analysis of high quality RNA, terpene synthase enzyme activity and terpenoid metabolites from resin ducts and cambial zone tissue of white spruce (Picea glauca). BMC Plant Biol 10:106
Alfaro R (1995) An induced defense reaction in white spruce to attack by the white pine weevil, Pissodes strobi. Can J For Res 25:1725–1730
Brignolas F, Lieutier F, Sauvard D, Christiansen E, Berryman AA (1998) Phenolic predictors for Norway spruce resistance to the bark beetle Ips typographus (Coleoptera: Scolytidae) and an associated fungus, Ceratocystis polonica. Can J For Res 28:720–728
Burke C, Croteau R (2002) Geranyl diphosphate synthase from Abies grandis: cDNA isolation, functional expression, and characterization. Arch Biochem Biophys 405:130–136
Byun-McKay A, Godard A, Toudefallah M, Martin M, Alfaro R, King J, Bohlmann J, Plant AL (2006) Wound-induced terpene synthase gene expression in Sitka spruce that exhibit resistance or susceptibility to attack by the white pine weevil. Plant Physiol 140:1009–1021
Christiansen E, Krokene P, Berryman AA, Franceschi VR, Krekling T, Lieutier F, Lönneborg A, Solheim H (1999) Mechanical injury and fungal infection induce acquired resistance in Norway spruce. Tree Physiol 19:399–403
Dong X (2004) NPR1, all things considered. Curr Opin Plant Biol 7:547–552
Erbilgin N, Krokene P, Christiansen E, Zeneli G, Gershenzon J (2006) Exogenous application of methyl jasmonate elicits defenses in Norway spruce (Picea abies) and reduces host colonization by the bark beetle Ips typographus. Oecologia 148:426–436
Fäldt J, Martin D, Miller B, Rawat S, Bohlmann J (2003) Traumatic resin defense in Norway spruce (Picea abies): methyl jasmonate-induced terpene synthase gene expression, and cDNA cloning and functional characterization of (+)-3-carene synthase. Plant Mol Biol 51:119–133
Fobert PR, Despres C (2005) Redox control of systemic acquired resistance. Curr Opin Plant Biol 8:378–382
Fonseca S, Chini A, Hamberg M, Adie B, Porzel A, Kramell R, Miersch O, Wasternack C, Solano R (2009) (+)-7-Iso-Jasmonoyl-l-isoleucine is the endogenous bioactive jasmonate. Nat Chem Biol 5:344–350
Franceschi VR, Krokene P, Krekling T, Christiansen E (2000) Phloem parenchyma cells are involved in local and distant defense responses to fungal inoculation or bark-beetle attack in Norway spruce (Pinaceae). Am J Bot 87:314–326
Franceschi VR, Krekling T, Christiansen E (2002) Application of methyl jasmonate on Picea abies (Pinaceae) stems induces defense-related responses in phloem and xylem. Am J Bot 89:578–586
Franceschi VR, Krokene P, Christiansen E, Krekling T (2005) Anatomical and chemical defenses of conifer bark against bark beetles and other pests. New Phytol 167:353–375
Gershenzon J, Kreis JW (1999) Biochemistry of terpenoids: monoterpenes, sesquiterpenes, diterpenes, sterols, cardiac glycosides, and steroid saponins. In: Wink M (ed) Biochemistry of plant secondary metabolism, annual plant reviews, vol 21. Sheffield Academic Press, Sheffield, pp 222–299
Hall DE, Robert JA, Keeling CI, Domanski D, Quesada AL, Jancsik S, Kuzyk MA, Hamberger B, Borchers CH, Bohlmann J (2011) An integrated genomic, proteomic and biochemical analysis of (+)-3-carene biosynthesis in Sitka spruce (Picea sitchensis) genotypes that are resistant or susceptible to white pine weevil. Plant J 65:936–948
Hamberger B, Bohlmann J (2006) Cytochrome P450 mono-oxygenases in conifer genomes: discovery of members of the terpenoid oxygenase superfamily in spruce and pine. Biochem Soc Trans 34:1209–1214
Heijari J, Nerg AM, Kainulainen P, Viiri H, Vuorinen M, Holopainen JK (2005) Application of methyl jasmonate reduces growth but increases chemical defence and resistance against Hylobius abietis in Scots pine seedlings. Ent Exp Appl 115:117–124
Hsieh FL, Chang TH, Ko TP, Wang AH (2011) Structure and mechanism of an Arabidopsis medium/long-chain-length prenyl pyrophosphate synthase. Plant Physiol 155:1079–1090
Huber DPW, Ralph S, Bohlmann J (2004) Genomic hardwiring and phenotypic plasticity of terpenoid-based defenses in conifers. J Chem Ecol 30:2399–2418
Huber DP, Philippe RN, Godard KA, Sturrock RN, Bohlmann J (2005) Characterization of four terpene synthase cDNAs from methyl jasmonate-induced Douglas-fir, Pseudotsuga menziesii. Phytochemistry 66:1427–1439
Hudgins JW, Christiansen E, Franceschi VR (2004) Induction of anatomically based defense responses in stems of diverse conifers by methyl jasmonate: a phylogenetic perspective. Tree Physiol 24:251–264
Hudgins JW, Franceschi VR (2004) Methyl jasmonate-induced ethylene production is responsible for conifer phloem defense responses and reprogramming of stem cambial zone for traumatic resin duct formation. Plant Physiol 135:2134–2149
Hudgins JW, Christiansen E, Franceschi VR (2003) Methyl jasmonate induces changes mimicking anatomical defenses in diverse members of the Pinaceae. Tree Physiol 23:361–371
Hudgins JW, Ralph SG, Franceschi VR, Bohlmann J (2006) Ethylene in induced conifer defense: cDNA cloning, protein expression, and cellular and subcellular localization of 1-aminocyclopropane-1-carboxylate oxidase in resin duct and phenolic parenchyma cells. Planta 224:865–877
Keeling CI, Bohlmann J (2006a) Diterpene resin acids in conifers. Phytochemistry 67:2415–2423
Keeling CI, Bohlmann J (2006b) Genes, enzymes and chemicals of terpenoid diversity in the constitutive and induced defence of conifers against insects and pathogens. New Phytol 170:657–675
Kozlowski G, Metraux JP (1998) Infection of Norway spruce (Picea abies (L.) Karst) seedlings with Pythium irregulare Buism and Pythium ultimum Trow: histological and biochemical responses. Eur J Plant Path 104:225–234
Kozlowski G, Buchala A, Metraux JP (1999) Methyl jasmonate protects Norway spruce Picea abies (L.) Karst. seedlings against Pythium ultimum Trow. Physiol Mol Plant Path 55:53–58
Krokene P, Nagy NE, Solheim H (2008a) Methyl jasmonate and oxalic acid treatment of Norway spruce: anatomically based defence responses and increased resistance against fungal infection. Tree Physiol 28:29–35
Krokene P, Nagy NE, Krekling T (2008b) Traumatic resin ducts and polyphenolic parenchyma cells in conifers. In: Schaller A (ed) Induced plant resistance to herbivory. Springer Science+Business Media, Berlin, pp 147–169
Martin D, Tholl D, Gershenzon J, Bohlmann J (2002) Methyl jasmonate induces traumatic resin ducts, terpenoid resin biosynthesis, and terpenoid accumulation in developing xylem of Norway spruce stems. Plant Physiol 129:1003–1018
Martin DM, Gershenzon J, Bohlmann J (2003) Induction of volatile terpene biosynthesis and diurnal emission by methyl jasmonate in foliage of Norway spruce. Plant Physiol 132:1586–1599
Martin DM, Fäldt J, Bohlmann J (2004) Functional characterization of nine Norway spruce TPS genes and evolution of gymnosperm terpene synthases of the TPS-d subfamily. Plant Physiol 135:1908–1927
Miller B, Madilao LL, Ralph S, Bohlmann J (2005) Insect-induced conifer defense. White pine weevil and methyl jasmonate induce traumatic resinosis, de novo formed volatile emissions, and accumulation of terpenoid synthase and putative octadecanoid pathway transcripts in Sitka spruce. Plant Physiol 137:369–382
Mueller MJ, Brodschelm W, Spannagl E, Zenk MH (1993) Signaling in the elicitation process is mediated through the octadecanoid pathway leading to jasmonic acid. Proc Natl Acad Sci USA 90:7490–7494
Nagy NE, Franceschi VR, Solheim H, Krekling T, Christiansen E (2000) Wound-induced traumatic resin duct development in stems of Norway spruce (Pinaceae): anatomy and cytochemical traits. Am J Bot 87:302–313
Ouaked F, Rozhon W, Lecourieux D, Hirt H (2003) A MAPK pathway mediates ethylene signaling in plants. EMBO 22:1282–1288
Peters WJ (1977) Ethrel-bipyridilium synergism in slash pine. In: Proceedings of the fourth lightwood research coordinating council, annual meeting, 18–19 Jan 1977. Energy Research and Development Program, pp 78–83
Phillips MA, Bohlmann J, Gershenzon J (2006) Molecular regulation of included terpenoid biosynthesis in conifers. Phytochem Rev 5:179–189
Phillips MA, Walter MH, Ralph SG, Dabrowska P, Luck K, Urós EM, Boland W, Strack D, Rodríguez-Concepción M, Bohlmann J, Gershenzon J (2007) Functional identification and differential expression of 1-deoxy-d-xylulose 5-phosphate synthase in induced terpenoid resin formation of Norway spruce (Picea abies). Plant Mol Biol 65:243–257
Popp M, Johnson JD, Lesney M (1995) Changes in ethylene production and monoterpene concentration in slash pine and loblolly pine following inoculation with bark beetle vectored fungi. Tree Physiol 15:807–812
Ralph SG, Yueh H, Friedmann M, Aeschliman D, Zeznik JA, Nelson CC, Butterfield YSN, Kirkpatrick R, Liu J, Jones SJM, Marra MA, Douglas CJ, Ritland K, Bohlmann J (2006) Conifer defence against insects: microarray gene expression profiling of Sitka spruce (Picea sitchensis) induced by mechanical wounding or feeding by spruce budworms (Choristoneurea occidentalis) or white pine weevils (Pissodes strobi) reveals large-scale changes of the host transcriptome. Plant Cell Environ 29:1545–1570
Ralph SG, Hudgins JW, Jancsik S, Franceschi VR, Bohlmann J (2007) Aminocyclopropane carboxylic acid synthase is a regulated step in ethylene-dependent induced conifer defense. Full-length cDNA cloning of a multigene family, differential constitutive, and wound- and insect-induced expression, and cellular and subcellular localization in spruce and Douglas fir. Plant Physiol 143:410–424
Ralph SG, Chun HJE, Kolosova N, Cooper D, Oddy C, Ritland CD, Kirkpatrick R, Moore R, Barber S, Holt RA, Jones SJM, Marra MA, Douglas CJ, Ritland K, Bohlmann J (2008) A conifer genomics resource of 200,000 spruce (Picea spp.) ESTs and 6,464 high-quality, sequence-finished full-length cDNAs for Sitka spruce (Picea sitchensis). BMC Genomics 9:484
Ro DK, Bohlmann J (2006) Diterpene resin acid biosynthesis in loblolly pine (Pinus taeda): Functional characterization of abietadiene/levopimaradiene synthase (PtTPS-LAS) cDNA and subcellular targeting of PtTPS-LAS and abietadienol/abietadienal oxidase (PtAO, CYP720B1). Phytochemistry 67:1572–1578
Rodriguez MC, Petersen M, Mundy J (2010) Mitogen-activated protein kinase signaling in plants. Annu Rev Plant Biol 61:621–649
Royo J, Vancanneyt G, Perez AG, Störmann K, Rosahl S, Sánchez-Serrano JJ (1996) Characterization of three potato lipoxygenases with distinct enzymatic activities and different organ-specific and wound-regulated expression patterns. J Biol Chem 271:21012–21019
Schmidt A, Gershenzon J (2007) Cloning and characterization of isoprenyl diphosphate synthases with farnesyl diphosphate and geranylgeranyl diphosphate synthase activity from Norway spruce (Picea abies) and their relation to induced oleoresin formation. Phytochemistry 68:2649–2659
Schmidt A, Gershenzon J (2008) Cloning and characterization of two different types of geranyl diphosphate synthases from Norway spruce (Picea abies). Phytochemistry 69:49–57
Schmidt A, Zeneli G, Hietala AM, Fossdal CG, Krokene P, Christiansen E, Gershenzon J (2005) Induced chemical defenses in conifers: biochemical and molecular approaches to studying their function. In: Romeo JT (ed) Chemical ecology and phytochemistry of forest ecosystems, vol 39. Elsevier, Tampa, pp 1–28
Schmidt L, Hummel GM, Schöttner M, Schurr U, Walter A (2010a) Jasmonic acid does not mediate root growth responses to wounding in Arabidopsis thaliana. Plant Cell Environ 33:104–116
Schmidt A, Wächtler B, Temp U, Krekling T, Séguin A, Gershenzon J (2010b) A bifunctional geranyl and geranylgeranyl diphosphate synthase is involved in terpene oleoresin formation in Norway spruce (Picea abies). Plant Physiol 152:639–655
Spoel SH, Koornneef A, Claessens SM, Korzelius JP, Van Pelt JA, Mueller MJ, Buchala AJ, Métraux JP, Brown R, Kazan K, Van Loon LC, Dong X, Pieterse CM (2003) NPR1 modulates cross-talk between salicylate- and jasmonate-dependent defense pathways through a novel function in the cytosol. Plant Cell 15:760–770
Takahashi F, Yoshida R, Ichimura K, Mizoguchi T, Seo S, Yonezawa M, Maruyama K, Yamaguchi-Shinozaki K, Shinozaki K (2007) The mitogen-activated protein kinase cascade MKK3-MPK6 is an important part of the jasmonate signal transduction pathway in Arabidopsis. Plant Cell 19:805–818
Tholl D, Croteau R, Gershenzon J (2001) Partial purification and characterization of the short-chain prenyltransferases, geranyl diphosphate synthase and farnesyl diphosphate synthase, from Abies grandis (grand fir). Arch Biochem Biophys 386:233–242
Tomlin ES, Alfaro RI, Borden JH, He FL (1998) Histological response of resistant and susceptible white spruce to simulated white pine weevil damage. Tree Physiol 18:21–28
Urbanek-Krajnc A, Kristl J, Ivancic A (2011) Application of salicylic acid induces antioxidant defense responses in the phloem of Picea abies and inhibits colonization by Ips typographus. For Ecol Manage 261:416–426
Wang KL, Li H, Ecker JR (2002) Ethylene biosynthesis and signaling networks. Plant Cell 14:131–151
Wasternack C (2007) Jasmonates: an update on biosynthesis, signal transduction and action in plant stress response, growth and development. Ann Bot 100:681–697
Wasternack C, Kombrink E (2010) Jasmonates: structural requirements for lipid-derived signals active in plant stress responses and development. ACS Chem Biol 5:63–77
Zeneli G, Krokene P, Christiansen E, Krekling T, Gershenzon J (2006) Methyl jasmonate treatment of large Norway spruce (Picea abies) trees increases the accumulation of terpenoid resin components and protects against infection by Ceratocystis polonica, a bark beetle-associated fungus. Tree Physiol 26:977–988
Zhao T, Krokene P, Björklund N, Långström B, Solheim H, Christiansen E, Borg-Karlson AK (2010) The influence of Ceratocystis polonica inoculation and methyl jasmonate application on terpene chemistry of Norway spruce, Picea abies. Phytochemistry 71:1332–1341
Zulak KG, Bohlmann J (2010) Terpenoid biosynthesis and specialized vascular cells of conifer defense. J Integr Plant Biol 52:86–97
Zulak KG, Lippert DN, Kuzyk MA, Domanski D, Chou T, Borchers CH, Bohlmann J (2009) Targeted proteomics using selected reaction monitoring reveals the induction of specific terpene synthases in a multi-level study of methyl jasmonate-treated Norway spruce (Picea abies). Plant J 60:1015–1030
Zulak KG, Dullat HK, Keeling CI, Lippert D, Bohlmann J (2010) Immunofluorescence localization of levopimaradiene/abietadiene synthase in methyl jasmonate treated stems of Sitka spruce (Picea sitchensis) shows activation of diterpenoid biosynthesis in cortical and developing traumatic resin ducts. Phytochemistry 71:1695–1699
Acknowledgments
We thank Marion Stäger and Elin Ørmen for technical assistance, Antje Burse for critical reading of the manuscript, Michael Reichelt for help in LC-MS analysis, and the Max-Planck-Society and the Norwegian Forest and Landscape Institute for financial support.
Open Access
This article is distributed under the terms of the Creative Commons Attribution Noncommercial License which permits any noncommercial use, distribution, and reproduction in any medium, provided the original author(s) and source are credited.
Author information
Authors and Affiliations
Corresponding author
Electronic supplementary material
Below is the link to the electronic supplementary material.
Rights and permissions
Open Access This is an open access article distributed under the terms of the Creative Commons Attribution Noncommercial License (https://creativecommons.org/licenses/by-nc/2.0), which permits any noncommercial use, distribution, and reproduction in any medium, provided the original author(s) and source are credited.
About this article
Cite this article
Schmidt, A., Nagel, R., Krekling, T. et al. Induction of isoprenyl diphosphate synthases, plant hormones and defense signalling genes correlates with traumatic resin duct formation in Norway spruce (Picea abies). Plant Mol Biol 77, 577–590 (2011). https://doi.org/10.1007/s11103-011-9832-7
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11103-011-9832-7