Abstract
Purpose
The regulatory effects of estradiol on pituitary homeostasis have been well documented. However, the expression patterns of ERα66 and ERα36 and their correlations with the clinical course of postoperative prolactinoma tumors remain unclear.
Methods
The expression of ERα36, ERα66, Ki67, p53, and CD31 were determined by immunohistochemistry in 62 prolactinoma patients. Snap-frozen tumors and normal pituitaries were also examined by western blotting for estrogen receptor detection.
Results
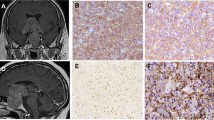
A broad expression of ERα36 was identified in normal pituitaries. The median scores of ERα36 and ERα66 expression were 8 and 6 in normal pituitaries and 4 and 0 in tumors, respectively. Four phenotypes of ERα36 and ERα66 expression were explored in tumors with regard to sex, invasiveness, dopamine resistance, and recurrence. Low ERα36 expression was associated with tumor invasion and increased Ki67. Low ERα66 expression was associated with tumor invasion, dopamine-agonist resistance, and enhanced tumor size. Multivariable logistic regression analysis showed that low ERα36 expression is an independent risk factor for invasiveness. The significant inverse association of ERα66 with invasiveness, dopamine resistance, and tumor size remained significant after adjustment for sex as a potential confounder. After controlling for sex, the low ERα66/low ERα36 phenotype was 6.24 times more prevalent in invasive tumors than in noninvasive tumors. Although the decreasing trend of CD31 expression from surrounding nontumoral lactotroph adenomas to tumors was similar to that of the estrogen receptors, a significant correlation was not observed here.
Conclusion
The decreasing trends of ERα36 and ERα66 expression from normal pituitaries to tumors are associated with aggressive clinical behavior.


Similar content being viewed by others
References
Melmed S, Casanueva FF, Hoffman AR, Kleinberg DL, Montori VM, Schlechte JA, Wass JA (2011) Diagnosis and treatment of hyperprolactinemia: an Endocrine Society clinical practice guideline. J Clin Endocrinol Metab 96:273–288. https://doi.org/10.1210/jc.2010-1692
Lopes MBS (2017) The 2017 World Health Organization classification of tumors of the pituitary gland: a summary. Acta Neuropathol 134:521–535. https://doi.org/10.1007/s00401-017-1769-8
Wang AT, Mullan RJ, Lane MA, Hazem A, Prasad C, Gathaiya NW, Fernández-Balsells MM, Bagatto A, Coto-Yglesias F, Carey J (2012) Treatment of hyperprolactinemia: a systematic review and meta-analysis. Syst Rev 1:33. https://doi.org/10.1186/2046-4053-1-33
Hu B, Mao Z, Jiang X, He D, Wang Z, Wang X, Zhu Y, Wang H (2018) Role of TGF-β1/Smad3-mediated fibrosis in drug resistance mechanism of prolactinoma. Brain Res 1698:204–212
Menucci M, Quiñones-Hinojosa A, Burger P, Salvatori R (2011) Effect of dopaminergic drug treatment on surgical findings in prolactinomas. Pituitary 14:68–74. https://doi.org/10.1007/s11102-010-0261-4
Ananthakrishnan S (2017) The dark side of dopamine agonist therapy in prolactinoma management. AACE Clin Case Rep 3(4):e384–e386
Roelfsema F, Biermasz NR, Pereira AM (2012) Clinical factors involved in the recurrence of pituitary adenomas after surgical remission: a structured review and meta-analysis. Pituitary 15:71–83. https://doi.org/10.1007/s11102-011-0347-7
Thomson JA, Gray CE, Teasdale GM (2002) Relapse of hyperprolactinemia after transsphenoidal surgery for microprolactinoma: lessons from long-term follow-up. Neurosurgery 50:36–40. https://doi.org/10.1097/00006123-200201000-00007
Ferraris J, Bernichtein S, Pisera D, Goffin V (2013) Use of prolactin receptor antagonist to better understand prolactin regulation of pituitary homeostasis. Neuroendocrinology 98:171–179. https://doi.org/10.1159/000354701
Heldring N, Pike A, Andersson S, Matthews J, Cheng G, Hartman J, Tujague M, Strom A, Treuter E, Warner M (2007) Estrogen receptors: how do they signal and what are their targets. Physiol Rev 87:905–931
Wang Z, Zhang X, Shen P, Loggie BW, Chang Y, Deuel TF (2006) A variant of estrogen receptor-α, hER-α36: transduction of estrogen- and antiestrogen-dependent membrane-initiated mitogenic signaling. Proc Natl Acad Sci USA 103:9063–9068. https://doi.org/10.1073/pnas.0603339103
Manoranjan B, Salehi F, Scheithauer B, Rotondo F, Kovacs K, Cusimano M (2010) Estrogen receptors α and β immunohistochemical expression: clinicopathological correlations in pituitary adenomas. Anticancer Res 30:2897–2904
Pereira-Lima JF, Marroni CP, Pizarro CB, Barbosa-Coutinho LM, Ferreira NP, Oliveira MC (2004) Immunohistochemical detection of estrogen receptor alpha in pituitary adenomas and its correlation with cellular replication. Neuroendocrinology 79:119–124. https://doi.org/10.1159/000077269
Delgrange E, Vasiljevic A, Wierinckx A, François P, Jouanneau E, Raverot G, Trouillas J (2015) Expression of estrogen receptor alpha is associated with prolactin pituitary tumor prognosis and supports the sex-related difference in tumor growth. Eur J Endocrinol 172:791–801. https://doi.org/10.1530/EJE-14-0990
Wang Q, Jiang J, Ying G, Xie X-Q, Zhang X, Xu W, Zhang X, Song E, Bu H, Ping Y-F (2018) Tamoxifen enhances stemness and promotes metastasis of ERα36+ breast cancer by upregulating ALDH1A1 in cancer cells. Cell Res 28:336. https://doi.org/10.1038/cr.2018.15
Shi L, Dong B, Li Z, Lu Y, Ouyang T, Li J, Wang T, Fan Z, Fan T, Lin B (2009) Expression of ER-α36, a novel variant of estrogen receptor α, and resistance to tamoxifen treatment in breast cancer. J Clin Oncol 27:3423. https://doi.org/10.1200/JCO.2008.17.2254
Zheng Y, Zhang J, Xu Z, Sheng J, Zhang X, Wang H, Teng X, Liu X, Cao J, Teng L (2010) Quantitative profiles of the mRNAs of ER-α and its novel variant ER-α36 in breast cancers and matched normal tissues. J Zhejiang Univ Sci B 11:144–150. https://doi.org/10.1631/jzus.B0900266
Pelekanou V, Notas G, Kampa M, Tsentelierou E, Radojicic J, Leclercq G, Castanas E, Stathopoulos EN (2012) ERα36, a new variant of the ERα is expressed in triple negative breast carcinomas and has a specific transcriptomic signature in breast cancer cell lines. Steroids 77:928–934. https://doi.org/10.1016/j.steroids.2011.12.016
Deng H, Huang X, Fan J, Wang L, Xia Q, Yang X, Wang Z, Liu L (2010) A variant of estrogen receptor-α, ER-α36 is expressed in human gastric cancer and is highly correlated with lymph node metastasis. Oncol Rep 24:171–176
Dai Y-J, Qiu Y-B, Jiang R, Xu M, Zhao L, Chen GG, Liu Z-M (2017) Concomitant high expression of ERα36, EGFR and HER2 is associated with aggressive behaviors of papillary thyroid carcinomas. Sci Rep 7:12279. https://doi.org/10.1038/s41598-017-12478-1
Wang Q, Zhang W, Yang J, Liu Y-L, Yan Z-X, Guo Z-J, Li Y-J, Bian X-W (2015) High ERα36 Expression level and membrane location predict poor prognosis in renal cell carcinoma. Medicine.https://doi.org/10.1097/MD.0000000000001048
Schwartz N, Chaudhri RA, Hadadi A, Schwartz Z, Boyan BD (2014) 17Beta-estradiol promotes aggressive laryngeal cancer through membrane-associated estrogen receptor-alpha 36. Horm Cancer 5:22–32. https://doi.org/10.1007/s12672-013-0161-y
Turner HE, Harris AL, Melmed S, Wass JA (2003) Angiogenesis in endocrine tumors. Endocr Rev 24:600–632. https://doi.org/10.1210/er.2002-0008
Turner HE, Nagy Z, Gatter KC, Esiri MM, Harris AL, Wass JA (2000) Angiogenesis in pituitary adenomas and the normal pituitary gland. J Clin Endocrinol Metab 85:1159–1162
Cristina C, Luque GM, Demarchi G, Lopez Vicchi F, Zubeldia-Brenner L, Perez Millan MI, Perrone S, Ornstein AM, Lacau-Mengido IM, Berner SI (2014) Angiogenesis in pituitary adenomas: human studies and new mutant mouse models. Int J Endocrinol.https://doi.org/10.1155/2014/608497
Dworakowska D, Grossman AB (2018) Aggressive and malignant pituitary tumours: state-of-the-art. Endocr Relat Cancer 25:R559–R575. https://doi.org/10.1530/ERC-18-0228
Molitch ME (2014) Management of medically refractory prolactinoma. J Neurooncol 117:421–428. https://doi.org/10.1007/s11060-013-1270-8
Remmele W, Hildebrand U, Hienz HA, Klein P-J, Vierbuchen M, Behnken LJ, Heicke B, Scheidt E (1986) Comparative histological, histochemical, immunohistochemical and biochemical studies on oestrogen receptors, lectin receptors, and Barr bodies in human breast cancer. Virchows Arch A 409:127–147. https://doi.org/10.1007/bf00708323
Weidner N (1995) Current pathologic methods for measuring intratumoral microvessel density within breast carcinoma and other solid tumors. Breast Cancer Res Treat 36:169–180. https://doi.org/10.1007/bf00666038
Wang Z, Zhang X, Shen P, Loggie BW, Chang Y, Deuel TF (2005) Identification, cloning, and expression of human estrogen receptor-α36, a novel variant of human estrogen receptor-α66. Biochem Biophys Res Commun 336:1023–1027. https://doi.org/10.1016/j.bbrc.2005.08.226
Ciccarelli A, Daly AF, Beckers A (2005) The epidemiology of prolactinomas. Pituitary 8:3–6
Schlechte J, Dolan K, Sherman B, Chapler F, Luciano A (1989) The natural history of untreated hyperprolactinemia: a prospective analysis. J Clin Endocrinol Metab 68:412–418. https://doi.org/10.1210/jcem-68-2-412
Weiss MH, Teal J, Gott P, Wycoff R, Yadley R, Apuzzo ML, Giannotta SL, Kletzky O, March C (1983) Natural history of microprolactinomas: six-year follow-up. Neurosurgery 12:180–183. https://doi.org/10.1227/00006123-198302000-00008
Gürlek A, Karavitaki N, Ansorge O, Wass JA (2007) What are the markers of aggressiveness in prolactinomas? Changes in cell biology, extracellular matrix components, angiogenesis and genetics. Eur J Endocrinol 156:143–153. https://doi.org/10.1530/eje.1.02339
Khare S, Lila AR, Patt H, Yerawar C, Goroshi M, Bandgar T, Shah NS (2016) Gender differences in macroprolactinomas: a single centre experience. Endocr Connect 5:20–27. https://doi.org/10.1530/EC-15-0105
Delgrange E, Trouillas J, Maiter D, Donckier J, Tourniaire J (1997) Sex-related difference in the growth of prolactinomas: a clinical and proliferation marker study. J Clin Endocrinol Metab 82:2102–2107. https://doi.org/10.1210/jcem.82.7.4088
Nishioka H, Haraoka J, Akada K (2003) Growth potential of prolactinomas in men: is it really different from women? Surg Neurol 59:386–390. https://doi.org/10.1016/s0090-3019(03)00012-0
Colao A, Sarno AD, Cappabianca P, Briganti F, Pivonello R, Somma CD, Faggiano A, Biondi B, Lombardi G (2003) Gender differences in the prevalence, clinical features and response to cabergoline in hyperprolactinemia. Eur J Endocrinol 148:325–331. https://doi.org/10.1530/eje.0.1480325
Lv L, Zhang B, Wang M, Yin S, Zhou P, Hu Y, Zhang S, Chen C, Zhang N, Jiang S (2018) Invasive pituitary adenomas with gross total resection: the wait-and-see policy during postoperative management. J Clin Neurosci.https://doi.org/10.1016/j.jocn.2018.10.065
Lasolle H, Ilie MD, Raverot G (2019) Aggressive prolactinomas: how to manage. Pituitary.https://doi.org/10.1007/s11102-019-01000-7
Heaney AP, Fernando M, Melmed S (2002) Functional role of estrogen in pituitary tumor pathogenesis. J Clin Investig 109:277–283. https://doi.org/10.1172/JCI14264
Turner H, Nagy Z, Gatter K, Esiri M, Wass J, Harris A (2000) Proliferation, bcl-2 expression and angiogenesis in pituitary adenomas: relationship to tumour behaviour. Br J Cancer 82:1441. https://doi.org/10.1054/bjoc.1999.1074
Niveiro M, Aranda FI, Peiró G, Alenda C, Picó A (2005) Immunohistochemical analysis of tumor angiogenic factors in human pituitary adenomas. Hum Pathol 36:1090–1095. https://doi.org/10.1016/j.humpath.2005.07.015
Turner H, Nagy Z, Gatter K, Esiri M, Harris A, Wass J (2000) Angiogenesis in pituitary adenomas—relationship to endocrine function, treatment and outcome. J Endocrinol 165:475–482. https://doi.org/10.1677/joe.0.1650475
Cristina C, Perez-Millan MI, Luque G, Dulce RA, Sevlever G, Berner SI, Becu-Villalobos D (2010) VEGF and CD31 association in pituitary adenomas. Endocr Pathol 21:154–160. https://doi.org/10.1007/s12022-010-9119-6
Doane A, Danso M, Lal P, Donaton M, Zhang L, Hudis C, Gerald W (2006) An estrogen receptor-negative breast cancer subset characterized by a hormonally regulated transcriptional program and response to androgen. Oncogene 25:3994. https://doi.org/10.1038/sj.onc.1209415
Huang B, Omoto Y, Iwase H, Yamashita H, Toyama T, Coombes RC, Filipovic A, Warner M, Gustafsson J-Å (2014) Differential expression of estrogen receptor α, β1, and β2 in lobular and ductal breast cancer. Proc Natl Acad Sci USA 111:1933–1938. https://doi.org/10.1073/pnas.1323719111
Magri ML, Gottardo MF, Zárate S, Eijo G, Ferraris J, Jaita G, Ayala MM, Candolfi M, Pisera D, Seilicovich A (2016) Opposite effects of dihydrotestosterone and estradiol on apoptosis in the anterior pituitary gland from male rats. Endocrine 51:506–516. https://doi.org/10.1007/s12020-015-0719-2
Zárate S, Seilicovich A (2010) Estrogen receptors and signaling pathways in lactotropes and somatotropes. Neuroendocrinology 92:215–223. https://doi.org/10.1159/000321683
Radl DB, Zárate S, Jaita G, Ferraris J, Zaldivar V, Eijo G, Seilicovich A, Pisera D (2008) Apoptosis of lactotrophs induced by D2 receptor activation is estrogen dependent. Neuroendocrinology 88:43–52. https://doi.org/10.1159/000116117
Kang L, Zhang X, Xie Y, Tu Y, Wang D, Liu Z, Wang Z-Y (2010) Involvement of estrogen receptor variant ER-α36, not GPR30, in nongenomic estrogen signaling. Mol Endocrinol 24:709–721. https://doi.org/10.1210/me.2009-0317
Chen J-R, Plotkin LI, Aguirre JI, Han L, Jilka RL, Kousteni S, Bellido T, Manolagas SC (2005) Transient versus sustained phosphorylation and nuclear accumulation of ERKs underlie anti-versus pro-apoptotic effects of estrogens. J Biol Chem 280:4632–4638. https://doi.org/10.1074/jbc.M411530200
Wang Z-Y, Yin L (2015) Estrogen receptor alpha-36 (ER-α36): a new player in human breast cancer. Mol Cell Endocrinol 418:193–206. https://doi.org/10.1016/j.mce.2015.04.017
Kansra S, Yamagata S, Sneade L, Foster L, Ben-Jonathan N (2005) Differential effects of estrogen receptor antagonists on pituitary lactotroph proliferation and prolactin release. Mol Cell Endocrinol 239:27–36. https://doi.org/10.1016/j.mce.2005.04.008
Acknowledgements
The authors thank Dr. Hamideh Akbari and Dr. Nader Akbari Dilamghani for assistance with the collection of patient data and samples, Miss Fatemeh Abbaszadeh for her technical assistance and Dr. Yadollah Mehrabi for assistance with the statistical analysis.
Funding
This study was performed as a Ph.D. Thesis Project of Fatemeh Mahboobifard and was funded by the School of Medicine and Neuroscience Research Center (Grant No. A-A-725.1-1395) of Shahid Beheshti University of Medical Sciences, Iran.
Author information
Authors and Affiliations
Contributions
FM: Conception and design, literature search, performing the research, analysis and interpretation, writing the article. FB-Z: Evaluating the pathological sections and scoring the extent of staining. ZD: Collecting the follow-up information and revising the paper. MP: Evaluating the pathological sections and scoring the extent of staining. LD: Technical or logistic support, and revising the paper. MHP: Conception and design, revising the paper. GS: Collecting the patients’ samples. NI: Assistance with the statistical analysis. MJ: Supervision, conception and design, technical or logistic support, and revising the paper. All authors read and approved the final manuscript.
Corresponding author
Ethics declarations
Conflict of interest
The authors declare that they have no conflict of interest.
Ethical approval
The study was approved by the Ethics Committee of the Shahid Beheshti University of Medical Science (IR.SBMU.PHNS.REC 1395.74).
Informed consent
Informed consent was obtained from all patients.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Electronic supplementary material
Below is the link to the electronic supplementary material.
11102_2020_1029_MOESM1_ESM.pdf
Supplementary material 1 (PDF 687 kb). Supplementary Fig. 1 Immunohistochemical staining of ERα36 in a breast cancer core needle biopsy sample that was used as a positive control for ERα36 antibody. Strong positivity of ERα36 in the cytoplasm. a × 200 and b × 400. Supplementary Fig. 2 Immunohistochemical staining of ERα36 in normal pituitary tissue from the anterior pituitary gland prepared from the autopsy. More than 80% of cells show cytoplasmic expression of ERα36. a × 100 and b × 200. Supplementary Fig. 3 Immunohistochemical staining of ERα66 in two prolactinomas. a Eighty percent of cells show a strong nuclear positivity (score 12) × 400. b Sixty percent of cells show moderate nuclear positivity (score 6)
Rights and permissions
About this article
Cite this article
Mahboobifard, F., Bidari-Zerehpoosh, F., Davoudi, Z. et al. Expression patterns of ERα66 and its novel variant isoform ERα36 in lactotroph pituitary adenomas and associations with clinicopathological characteristics. Pituitary 23, 232–245 (2020). https://doi.org/10.1007/s11102-020-01029-z
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11102-020-01029-z