Abstract
Purpose
In overt hypercortisolism, growth hormone (GH) secretion is decreased and normalizes after surgery. In subclinical hypercortisolism (SH), GH secretion has been scarcely investigated. We assessed GH reserve in patients with and without SH and, in the former, also after recovery.
Methods
We enrolled 24 patients with adrenal adenomas, 12 with SH (SH+, 8 females, 58.3 ± 6.5 years) and 12 without SH (SH−; 11 females, 61.8 ± 10.6 years). SH was diagnosed in the presence of ≥2 out of: 1 mg overnight dexamethasone suppression test >83 nmol/L, urinary free cortisol (UFC) >193 nmol/day and ACTH levels <2.2 pmol/L. GH secretion was assessed by GHRH + Arginine test (GHRH–ARG) and age-adjusted serum IGF-I levels, expressed as SDS (IGF-I SDS). Eight SH+ patients were re-evaluated after the recovery from SH.
Results
Age, gender, body mass index (BMI) and IGF-I SDS were comparable between SH+ and SH− patients. After GHRH–ARG the mean GH peak levels (GH-P) and GH response (as Area Under Curve, GH-AUC) were lower in SH+ than in SH− patients (15.2 ± 8.1 vs 44.5 ± 30.9 μg/L, P = 0.004 and 1,418 ± 803 vs 4,028 ± 2,476 μg/L/120 min, P = 0.002, respectively), after adjusting for age and BMI. The GH-AUC and GH-P levels were negatively associated with UFC after adjusting for age and BMI (β = −0.39, P = 0.02 and β = −0.4, P = 0.020 respectively). After recovery, GH-P levels and GH-AUC increased as compared to baseline (23.7 ± 16.3 vs 15.8 ± 10.2 μg/L, P = 0.036 and 2,549 ± 1,982 vs 1,618 ± 911 μg/L/120 min, P = 0.012, respectively).
Conclusions
GH secretion reserve is decreased in SH patients and increases after the recovery.
Similar content being viewed by others
Background
It is known that the condition of overt endogenous (i.e. Cushing’s syndrome) or exogenous (chronic glucocorticoid treatment) hypercortisolism leads to a blunted or absent growth hormone (GH) secretion and response after a variety of stimuli [1–4]. A reduced hypothalamic GHRH secretion [3] and an enhanced somatostatin release [4, 5] together with a direct inhibition of pituitary somatotrophs [6] are the hypothesized mechanisms of the inhibitory effect exerted by glucocorticoids. In addition, the elevated blood glucose and free fatty acid concentrations, often associated with hypercortisolism, may influence GH secretion [7]. In adult patients with Cushing’s syndrome, GH secretion normalizes within a year after recovery, but a complete normalization of the cortisol secretion is needed to obtain a full restoration of GH reserve [8, 9].
Subclinical hypercortisolism (SH) is a status of altered cortisol secretion in the absence of the classical signs or symptoms of overt cortisol excess (i.e. purple striae, easy bruising, proximal muscle weakness and plethora) [10, 11]. Although poorly symptomatic, SH is associated with an increased prevalence of hypertension, diabetes and osteoporosis, similarly to what is observed in patients with Cushing’s syndrome [11–14]. The interest about SH is justified on the basis of its high prevalence that is estimated to be 5–30 % in patients with adrenal incidentalomas (AI), and between 0.2 and 2.0 % of the adult population [11, 15].
The available data about GH secretion in SH patients are scarce and discordant, probably due to the observational design of the studies and the different criteria used for diagnosing SH [16, 17]. In addition, no studies evaluated the changes of GH secretion in surgically treated AI patients after the recovery from SH. This is an important lack of knowledge, since in adults, the GH deficiency (GHD) is associated with abnormal body composition, insulin resistance, poor quality of life, adverse lipid and cardiovascular risk profile, and decreased bone mass [18, 19]. Therefore, a diminished GH secretion may contribute to the SH− related morbidity.
The aim of the present study was to evaluate GH secretory reserve in AI patients with and without SH and, in the former, also after successful surgery and restoration of a normal cortisol secretion.
Materials and methods
Subjects
Between October 2009 and October 2011, 105 consecutive AI patients referred to our Centre were evaluated for inclusion in the study (Fig. 1). Exclusion criteria were: (1) signs or symptoms of cortisol excess (i.e. moon facies, striae rubrae, skin atrophy or buffalo hump); (2) bilateral AI; (3) diseases and administration of drugs influencing GH, IGF-I and cortisol secretion or cortisol and dexamethasone metabolism (i.e. chronic renal failure, liver disease, alcoholism, depression, eating disorders, rheumatologic diseases, morbid obesity, hyperthyroidism, antiseizure drugs, thiazolidinediones); (4) other conditions or diseases potentially affecting GH secretion and/or adrenal gland function, such as male hypogonadism, history of malignant disease, congenital adrenal hyperplasia, infections, haemorrhage, pheochromocytoma, amyloidosis or infiltrative diseases, poorly compensated diabetes mellitus (i.e. glycated haemoglobin >53 mmol/mol). Among the 105 patients, 41 were excluded from the study because they felt in one or more of the exclusion criteria described above. Among the remaining 64 patients (13 SH+, 51 SH−), 11 (1 SH+, 10 SH−) refused to participate in the study. Therefore, 12 SH+ AI patients (8 females, 58.3 ± 6.5 years) were included in the study. From the remaining 41 SH− patients, the first 12 consecutive SH− AI patients (11 females, 61.8 ± 10.6 years) were enrolled.
Study flow diagram. Exclusion criteria: (1) signs or symptoms of cortisol excess (i.e. moon facies, striae rubrae, skin atrophy or buffalo hump); (2) bilateral AI; (3) diseases and administration of drugs influencing GH, IGF-I and cortisol secretion or cortisol and dexamethasone metabolism (i.e. chronic renal failure, liver disease, alcoholism, depression, eating disorders, rheumatologic diseases, morbid obesity, hyperthyroidism, antiseizure drugs, thiazolidinediones); (4) other conditions or diseases potentially affecting GH secretion and/or adrenal gland function, such as male hypogonadism, history of malignant disease, congenital adrenal hyperplasia, infections, haemorrhage, pheochromocytoma, amyloidosis or infiltrative diseases, poorly compensated diabetes mellitus (i.e. glycated haemoglobin >53 mmol/mol). AI adrenal incidentaloma, SH subclinical hypercortisolism. SH was diagnosed in the presence of at least 2 out of: 1 mg-DST >83 nmol/L, UFC >193 nmol/day and ACTH levels <2.2 pmol/L
All AI were discovered by abdominal ultrasound or computed tomography (CT) scan, performed for the evaluation of unrelated diseases. The finding of AI by ultrasound was confirmed with CT scan. At CT, all adrenal masses were unilateral, homogeneous and hypodense and with well-shaped features, consistent with the diagnosis of an adrenocortical adenoma. In all patients, the diagnosis of aldosteronoma was excluded by appropriate hormonal determinations (upright plasma renin activity and aldosterone).
Written informed consent was obtained from all subjects and the study was approved by our Ethics Committee.
Patients were defined as affected with SH in the presence of at least 2 of the 3 following criteria: serum cortisol levels at 8:00 a.m. after 1 mg dexamethasone overnight suppression test (1 mg-DST) >83 nmol/L, urinary free cortisol (UFC) levels >193 nmol/day (normal values: 27.6–193 nmol/day) and plasma adrenocorticotroph hormone (ACTH) at 8:00 a.m. <2.2 pmol/L. The use of a 1 mg-DST cut-off of >83 nmol/L rather than 138 nmol/L as recommended by the National Institutes of Health [20] was preferred to increase the test sensitivity [11]. In SH+ patients with ACTH levels between 1.1 and 4.4 pmol/L a corticotroph releasing hormone (CRH) stimulation test has been performed in order to confirm the ACTH-independent origin of SH. All tested patients had an ACTH peak post CRH stimulation test <6.6 pmol/L.
Methods
In all patients GH secretion was assessed by combined GHRH and arginine administration (GHRH–ARG) and basal serum IGF-I levels, expressed as SDS (IGF-I SDS, corrected for age). Body mass index (BMI) and insulin resistance index (HOMA-IR) were also evaluated.
Eight out of the 12 SH+ patients underwent unilateral adrenalectomy, the remaining four patients refused surgery. A precautionary steroid therapy with hydrocortisone 100 mg i.v., during the surgery, and, in seven out eight patients with cortisone acetate per os, immediately after the operation, was administered. The commonly used cortisone acetate dose was 25 mg/day, while higher dose of 37.5 mg/day were used in two obese patients. All patients took their daily dose of the drug in three divided doses, the greater on awakening to comply with the circadian rhythm of cortisol secretion. In patients who received replacement therapy with cortisone acetate, the mean duration of therapy was 11.1 months (range 1–27). After recovery from SH and at least 3 months after the complete restoration of a normal cortisol secretion, assessed by a 250 μg ACTH stimulation test, the GH reserve was re-evaluated in the eight patients operated on.
In all patients, at the study entry, plasma morning ACTH levels (mean of 3 determinations at 20-min intervals) were measured with a chemiluminescent immunometric assay (IMMULITE 2000, Siemens Medical Solutions Diagnostics, Los Angeles, CA), serum cortisol and UFC (after dichloromethane extraction) levels were determined with an electrochemiluminescence immunoassay “ECLIA” (Cobas, Roche Diagnostics GmbH, Mannheim, Germany). The intra- and inter-assay coefficients of variation were ≤10 % for ACTH, <2.8 % for serum cortisol and <4.7 % for UFC.
Serum GH was assayed with a chemiluminescent immunometric method (IMMULITE 2000, Siemens Medical Solutions Diagnostics, Los Angeles, CA) with a detection limit of 0.01 μg/L. The standards were calibrated to the first World Health Organization International Reference Preparation (code 80/505). After the second semester of 2010, the standards were calibrated to the WHO International Standard IS 98/574. Serum IGF-I levels were measured by a chemiluminescent immunometric assay (IMMULITE 2000, Siemens Medical Solutions Diagnostics, Los Angeles, CA), with an intra- and inter-assay coefficient of variation of 2.9 and 7.4 %, respectively. The values were compared with those from an appropriate age- and sex-adjusted range.
Dynamic GH secretion was investigated, after an overnight fast, by GHRH plus arginine test (GHRH 1–29; GEREF, Serono, Italy; 1 μg/kg i.v. at 0 min; arginine hydrochloride, 0.5 g/kg, up to a maximum of 30 g, i.v. over 30 min (from 0 to 30 min). Blood samples for GH evaluation were taken at 0, 15, 30, 45, 60, 90 and 120 min. Severe GHD was defined as a peak response of serum GH less than 11 μg/L in normal weight subjects (BMI <25 kg/m2), less than 8 μg/L in overweight subjects (BMI between 25 and 29.9 kg/m2) and less than 4.0 μg/L in obese subjects (BMI >30 kg/m2) [21].
Insulin resistance and sensitivity were calculated using Homeostasis Model Assessment-Insulin Resistance (HOMA-IR) [22].
Body mass index (BMI) was calculated as weight in kilograms divided by the square of height in meters.
All the other biochemical parameters were measured by standard procedures.
Statistical analysis and design of the study
Statistical analysis was performed by SPSS version 18.0 statistical package (SPSS Inc, Chicago, IL, USA). The results are expressed as mean ± SD.
The normality of distribution was tested by Kolmogorov–Smirnov test. The comparison of continuous variables between Group SH+ and Group SH− was performed using student t test or Mann–Whitney U test as appropriate. Categorical variables were compared by χ2 test.
The GH response to stimulus, measured as area under curve (GH-AUC), was calculated by trapezoidal integration. The general linear modelling was used for comparing the GH-peak and the GH-AUC after GHRH–ARG between SH+ and SH− Group and between the SH+ patients at baseline and post-adrenalectomy after adjusting for age and BMI.
The associations between the GH secretory reserve parameters and the different indexes of cortisol secretion were tested by either Pearson product moment correlation or Spearman correlation as appropriate. The multivariate linear regression analysis was performed to evaluate the associations between the GH secretion parameters and the indexes of cortisol secretion after adjusting for age and BMI.
P values of less than 0.05 were considered significant.
Results
GH secretion reserve in SH− and SH+ patients at baseline
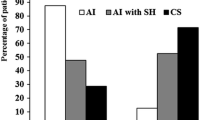
The clinical and hormonal characteristics of SH− and SH+ patients at baseline are reported in Table 1. Age, gender, BMI and HOMA-IR were comparable between the two groups. As expected, 1 mg-DST and UFC levels were higher, while ACTH levels lower in SH+ than in SH− patients. As far as GH response to GHRH–ARG was concerned, the mean GH peak levels (GH-P) and GH-AUC values were significantly lower in SH+ than in SH− patients, after adjusting for age and BMI by general linear modelling (15.2 ± 8.1 vs 44.5 ± 30.9 μg/L, P = 0.004 and 1,418 ± 803 vs 4,028 ± 2,476 μg/L/120 min, P = 0.002, respectively) (Fig. 2). No differences in IGF-I SDS and mean basal GH values were found between the two groups. Three patients, one SH− and two SH+, had an impaired response to GHRH–ARG (GH-P 5.2 μg/L, 5.4 μg/L and 7.7 μg/L, BMI 27.6, 25.6 and 28.2 respectively).
The bivariate correlation analysis showed that the GH-P and the GH-AUC levels after GHRH–ARG were inversely correlated with UFC (r = −0.54, P = 0.006 and r = −0.49, P = 0.015 respectively) and, as expected, with BMI (r = −0.66, P < 0.001 and r = −0.67, P < 0.001 respectively). The GH-AUC values tended to be directly correlated with ACTH levels (r = 0.39, P = 0.06). No correlations were found between both GH-AUC and GH-P levels after GHRH–ARG and 1 mg-DST and between both IGF-I and IGF-I SDS levels and all parameters of cortisol secretion.
In the multivariate linear regression analysis, the GH-AUC values and the GH-P levels after GHRH–ARG were negatively associated with UFC after adjusting for age and BMI (β = −0.39, P = 0.02 and β = −0.4, P = 0.02 respectively).
GH secretion reserve after adrenalectomy in SH+ patients
As shown in Table 2 and Fig. 3, in SH+ patients after adrenalectomy the mean GH-P levels and the GH-AUC values after GHRH–ARG significantly increased in comparison with pre-treatment values (23.7 ± 16.3 vs 15.8 ± 10.2 μg/L, P = 0.036 and 2,549 ± 1,982 vs 1,618 ± 911 μg/L/120 min, P = 0.012, respectively). In keeping with this results, the IGF-I SDS levels tended to increase after adrenalectomy in SH+ patients, although without reaching the statistical significance. In patients operated on, no significant changes in BMI were observed from baseline (BMI pre-adrenalectomy 26.8 ± 4.8 kg/m2, BMI post-adrenalectomy 25.9 ± 4.4 kg/m2, P = 0.220).
Mean GH values post-GHRH + arginine test in SH+ patients before and after adrenalectomy. GH growth hormone; SH subclinical hypercortisolism; SH was diagnosed in the presence of at least 2 out of: 1 mg-DST >83 nmol/L, UFC >193 nmol/day and ACTH levels <2.2 pmol/L. *P = 0.040 and 0.010 for post-GHRH + arginine GH peak and GH-AUC (area under curve) in SH+ patients post-adrenalectomy vs pre-adrenalectomy respectively
The two patients with SH+, who did not respond to the stimulus with GHRH–ARG at baseline, regained a normal response post-adrenalectomy (GH-P pre-adrenalectomy vs post-adrenalectomy: 5.4 vs 11.5 μg/L and 7.7 vs 11.8 μg/L respectively), even after adjusting for BMI changes (BMI pre-adrenalectomy vs post adrenalectomy: 25.6 vs 23.8 kg/m2 and 28.2 vs 26.3 kg/m2, respectively).
After adrenalectomy in SH+ patients all parameters of GH secretion were not statistically different to those found in SH− patients (Table 2).
Discussion
In the present case-control study we evaluated the GH reserve in AI patients with SH in comparison with AI patients without SH, and in the former group also after recovery from SH. We found that GH reserve is decreased in SH+ patients, in relation to cortisol hypersecretion, and that it increases after the recovery from SH. Hence, our study shows that SH is associated with a reduced GH secretion, similarly to what happens in the condition of overt cortisol excess.
These findings are apparently in contrast with those of two previous studies who did not found an altered GH reserve in AI patients with SH [16, 17]. In the former study, AI patients demonstrated a blunted GH response to GHRH, compared to those of healthy controls, that could be restored to normal by pretreatment with arginine, but, at variance with the present study, GH secretion reserve was not different between AI patients with and without SH [16]. However in this study the GH reserve was associated with cortisol secretion and the GH peak after GHRH tended to be lower in SH+ than in SH− patients (4.0 ± 2.2 vs 7.4 ± 7.0 μg/L, respectively), even though the statistical significance was not reached probably due to the small sample size (5 patients in each group).
In the second study, the presence of abnormal GH secretion was confirmed in the group of patients with Cushing’s syndrome but not in the group of patients with SH, as diagnosed on the basis of standard low dose dexamethasone suppression test (LDDST) only [17]. However, even in this study, the 4 AI patients with ascertained SH (i.e. 1 mg-DST >138 nmol/L) showed a reduced GH reserve as compared with AI patients without SH (18.5 ± 2.0 vs 30.2 ± 9.7 μg/L, respectively), without statistical significance. On the other hand, when using a more sensitive, and therefore less specific, cut-off of LDDST for diagnosing SH (i.e. cortisol ≥70 nmol/L), these authors were not able to find differences in GH secretion between AI patients with and without SH.
Indeed, to date, the diagnosis of SH remains a challenge for the physicians, due to the fact that in AI patients cortisol secretion may fluctuate over time and that the reliability of the parameters of HPA axis activity used for diagnosing a subtle hypercortisolism is not completely satisfactory [11].
In order to overcome these limitations, in the present study, for the first time, the GH reserve has been assessed also after recovery from SH. The increase of GH secretion reserve after the successful surgery further demonstrates that even a slight cortisol excess exerts an inhibitory effect on GH secretion. This is in keeping with emerging data, suggesting that SH has a negative effect on the glycometabolic control, body weight, hypertension, bone mass and quality [11, 13, 23–25]. Therefore, given the known important role of the GH/IGF-I axis on glucose, body composition, blood pressure and bone metabolism [18, 19], the present results of an impaired GH secretion in SH patients is an important pathophysiological finding. In our series only two SH+ patients had a subnormal response to GHRH–ARG, and were diagnosed to be affected by severe GHD, that is known to take benefit from recombinant human GH (rhGH) therapy [19]. However, it must be observed that, to date, the strict threshold used for the biochemical definition of severe GHD may lead to miss a subgroup of patients with partial GHD, that has been shown to be associated with abnormalities of body composition as in severe GHD [18]. Thus, a reduced activity of the GH/IGF-I axis, even if not below the cut-offs for defining severe GHD, could contribute to the metabolic derangements, increased cardiovascular risk and bone damage, which have been suggested to be present in AI patients with SH [11–14]. Unfortunately, to date, the possible clinical implications of this mildly diminished GH reserve and the effect of its treatment with recombinant human are unknown.
Besides the pathophysiological implications, these findings may have some clinical consequences. Firstly, in AI patients an impaired GH reserve should be considered among the possible complications of SH. On the other hand, the evaluation of GH secretion could be used as an additional parameter for the diagnosis of SH. Certainly, it would be impractical to perform a GHRH–ARG test in every patient with SH, but the presence of a blunted GH response to GHRH–ARG in a patient with a possible or doubtful SH can be considered as an extra criteria, along with the classic parameter of cortisol secretion, to help the physician to make the diagnosis. Further studies are needed to define the GH cut-off levels that should be considered as abnormal in this clinical setting.
The limitations of our study are related to the fact that, due to budget restrictions, the number of patients enrolled was small and we could not confirm our findings with the gold standard procedure insulin tolerance test (ITT). However, the GHRH–ARG test is considered a reliable tool to investigate the GH secretory reserve and it is currently used for clinical purposes [19]. In addition, considering the possible duration of the functional GH deficiency described after remission of Cushing’s syndrome [8], it is not possible to exclude that in the present study some patients have been re-evaluated too early after the cure of SH and that, therefore, the GH secretion recovery observed at the time of retesting was incomplete. Finally, one could hypothesize that the decrease in the mean BMI levels after the recovery from SH (even if slight and statistically not significant) may have influenced GH secretion. However, the difference found in the GH-peak and the GH-AUC levels after GHRH–ARG in SH+ patients between baseline and post-adrenalectomy were adjusted for BMI levels by General Linear Modelling. Therefore, the restoration of the GH secretion after the recovery from SH should be considered independent of the BMI changes.
In conclusion, the present study suggests that SH is associated with a blunted GH secretion that increases after the recovery. Further studies with a larger series of patients are needed to confirm the possible clinical applications of these findings.
References
Burguera B, Muruais C, Peñalva A, Dieguez C, Casanueva FF (1990) Dual and selective actions of glucocorticoids upon basal and stimulated growth hormone release in man. Neuroendocrinology 51:51–58
Smals AE, Pieters GF, Smals AG, Benraad TJ, Kloppenborg PW (1986) Human pancreatic growth hormone releasing hormone fails to stimulate human growth hormone both in Cushing’s disease and in Cushing’s syndrome due to adrenocortical adenoma. Clin Endocrinol (Oxf) 24:401–407
Leal-Cerro A, Pumar A, Garcia–Garcia E, Dieguez C, Casanueva FF (1994) Inhibition of growth hormone release after the combined administration of GHRH and GHRP-6 in patients with Cushing’s syndrome. Clin Endocrinol (Oxf) 41:649–654
Mazziotti G, Giustina A (2013) Glucocorticoids and the regulation of growth hormone secretion. Nat Rev Endocrinol. doi:10.1038/nrendo.2013.5
Wehrenberg WB, Bergman PJ, Stagg L, Ndon J, Giustina A (1990) Glucocorticoid inhibition of growth in rats: partial reversal with somatostatin antibodies. Endocrinology 127:2705–2708
Leal-Cerro A, Soto A, Martínez MA, Alvarez P, Isidro L, Casanueva FF, Dieguez C, Cordido F (2002) Effect of withdrawal of somatostatin plus growth hormone (GH)-releasing hormone as a stimulus of GH secretion in Cushing’s syndrome. Clin Endocrinol (Oxf) 57:745–749
Giustina A, Veldhuis JD (1998) Pathophysiology of the neuroregulation of growth hormone secretion in experimental animals and the human. Endocr Rev 19:717–797
Tzanela M, Karavitaki N, Stylianidou C, Tsagarakis S, Thalassinos NC (2004) Assessment of GH reserve before and after successful treatment of adult patients with Cushing’s syndrome. Clin Endocrinol (Oxf) 60:309–314
Mukherjee A, Murray RD, Teasdale GM, Shalet SM (2004) Acquired prolactin deficiency (APD) after treatment for Cushing’s disease is a reliable marker of irreversible severe GHD but does not reflect disease status. Clin Endocrinol (Oxf) 60:476–483
Boscaro M, Barzon L, Fallo F, Sonino N (2001) Cushing’s syndrome. Lancet 357:783–791
Chiodini I (2011) Clinical review: diagnosis and treatment of subclinical hypercortisolism. J Clin Endocrinol Metab 96:1223–1236
Terzolo M, Pia A, Alì A, Osella G, Reimondo G, Bovio S, Daffara F, Procopio M, Paccotti P, Borretta G, Angeli A (2002) Adrenal incidentaloma: a new cause of the metabolic syndrome? J Clin Endocrinol Metab 87:998–1003
Chiodini I, Morelli V, Masserini B, Salcuni AS, Eller-Vainicher C, Viti R, Coletti F, Guglielmi G, Battista C, Carnevale V, Iorio L, Beck-Peccoz P, Arosio M, Ambrosi B, Scillitani A (2009) Bone mineral density, prevalence of vertebral fractures and bone quality in patients with adrenal incidentalomas with and without subclinical hypercortisolism: an Italian Multicenter Study. J Clin Endocrinol Metab 94:3207–3214
Chiodini I, Torlontano M, Scillitani A, Arosio M, Bacci S, Di Lembo S, Epaminonda P, Augello G, Enrini R, Ambrosi B, Adda G, Trischitta V (2005) Association of subclinical hypercortisolism with type 2 diabetes mellitus: a case-control study in hospitalized patients. Eur J Endocrinol 15:837–844
Terzolo M, Osella G, Alì A, Borretta G, Cesario F, Paccotti P, Angeli A (1998) Subclinical Cushing’s syndrome in adrenal incidentaloma. Clinical Endocrinol (Oxf) 48:89–97
Terzolo M, Bossoni S, Alí A, Doga M, Reimondo G, Milani G, Peretti P, Manelli F, Angeli A, Giustina A (2000) Growth hormone (GH) responses to GH-releasing hormone alone or combined with arginine in patients with adrenal incidentaloma: evidence for enhanced somatostatinergic tone. J Clin Endocrinol Metab 85:1310–1315
Tzanela M, Zianni D, Stylianidou Ch, Karavitaki N, Tsagarakis S, Thalassinos NC (2005) Evaluation of GH reserve in patients with adrenal incidentalomas and biochemical evidence of subclinical autonomous glucocorticoid hypersecretion. Clin Endocrinol (Oxf) 62:597–602
Murray RD, Adams JE, Shalet SM (2004) Adults with partial growth hormone deficiency have an adverse body composition. J Clin Endocrinol Metab 89:1586–1591
Molitch ME, Clemmons DR, Malozowski S, Merriam GR, Vance ML (2011) Evaluation and treatment of adult growth hormone deficiency: an Endocrine Society clinical practice guideline. J Clin Endocrinol Metab 96:1587–1609
NIH state-of-the-science statement on management of the clinically inapparent adrenal mass (“incidentaloma”) (2002) NIH Consens. State Sci Statements 19: 1–25
Ho KK (2007) 2007 GH deficiency consensus workshop participants consensus guidelines for the diagnosis and treatment of adults with GH deficiency II: a statement of the GH research society in association with the European society for Pediatric Endocrinology, Lawson Wilkins society, European Society of Endocrinology, Japan Endocrine Society, and Endocrine Society of Australia. Eur J Endocrinol 157:695–700
Matthews DR, Hosker JP, Rudenski AS, Naylor BA, Treacher DF, Turner RC (1985) Homeostasis model assessment: insulin resistance and beta-cell function from fasting plasma glucose and insulin concentration in men. Diabetologia 28:412–419
Toniato A, Merante-Boschin I, Opocher G, Pelizzo MR, Schiavi F, Ballotta E (2009) Surgical versus conservative management for subclinical Cushing syndrome in adrenal incidentalomas: a prospective randomized study. Ann Surg 249:388–391
Chiodini I, Morelli V, Salcuni AS, Eller-Vainicher C, Torlontano M, Coletti F, Iorio L, Cuttitta A, Ambrosio A, Vicentini L, Pellegrini F, Copetti M, Beck-Peccoz P, Arosio M, Ambrosi B, Trischitta V, Scillitani A (2010) Beneficial metabolic effects of prompt surgical treatment in patients with an adrenal incidentaloma causing biochemical hypercortisolism. J Clin Endocrinol Metab 95:2736–2745
Eller-Vainicher C, Morelli V, Ulivieri FM, Palmieri S, Zhukouskaya VV, Cairoli E, Pino R, Naccarato R, Scillitani A, Beck-Peccoz P, Chiodini I (2012) Bone quality as measured by trabecular bone score (TBS) in patients with adrenal incidentalomas with and without subclinical hypercortisolism. J Bone Miner Res 27:2223–2230
Conflict of interest
The authors declare that they have no conflict of interest.
Ethical Standards
The study was conducted in accordance with applicable laws.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Palmieri, S., Morelli, V., Salcuni, A.S. et al. GH secretion reserve in subclinical hypercortisolism. Pituitary 17, 470–476 (2014). https://doi.org/10.1007/s11102-013-0528-7
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11102-013-0528-7