Abstract
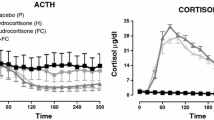
Mineralocorticoid receptors (MR) in the hippocampus display an important role in the control of hypothalamic–pituitary–adrenal (HPA)-axis, mediating the “proactive”-feedback of glucocorticoids. Fludrocortisone (FC), a potent MR agonist, has been shown to decrease HPA activity through a mechanism placed at hippocampal level. In order to clarify the effects of MR agonism on HPA function in humans, we studied the effects of FC, in a dose-related manner, on both basal and CRH-stimulated HPA axis during the quiescent phase. 8 young women were studied. ACTH, cortisol and aldosterone levels were evaluated every 15′, from 1600 to 2000 hours, in randomized sessions: (1) placebo p.o. + placebo i.v., (2) 0.3 mg FC p.o. + placebo, (3) 0.1 mg FC. + placebo, (4) 0.075 mg FC + placebo, (5) 0.05 mg FC + placebo, (6) placebo + hCRH (2.0 μg/kg iv-bolus), (7) 0.3 mg FC + hCRH, (8) 0.1 mg FC + hCRH, (9) 0.075 mg FC + hCRH, (10) 0.05 mg FC + hCRH. FC induced a dose-related trend toward a further decrease of the ACTH and cortisol levels, while it showed a significant and dose-dependent inhibition of the hormonal response to hCRH (p < 0.05 for the doses of 0.3, 0.1 and 0.075 mg). Conversely, 0.05 mg FC did not modify the CRH-stimulatory effect on both ACTH and cortisol secretion. Aldosterone levels were not modified by FC administration. Fludrocortisone inhibits corticotrope and adrenal response to hCRH in humans, in a dose-dependent manner. The 0.075 mg FC seems the lowest active while 0.05 mg the first neutral dose on HPA activity. These data suggest a possible hypophysial MR-mediated inhibiting effect of FC, although its pituitary glucocorticoid-mediated effect cannot be excluded. The interplay between fludrocortisone and hypophysial glucocorticoid receptors needs to be clarified in order to define better the clinical consequences of the hormonal replacement therapy of patients with primary adrenal insufficiency.


Similar content being viewed by others
References
Gaillard RC, Al-Damluji S (1987) Stress and the pituitary-adrenal axis. Baillieres Clin Endocrinol Metab 1(2):319–354
Orth DN (1992) Corticotropin-releasing hormone in humans. Endocr Rev 13:164–191
Jacobson L, Sapolsky R (1991) The role of the hippocampus in feedback regulation of the hypothalamic-pituitary-adrenocortical axis. Endocr Rev 12:118–134
De Kloet ER, Vreugdehil E, Oitzl MS, Joels M (1998) Brain corticosteroid receptor balance in health and disease. Endocr Rev 19(3):269–301
Arvat E, Maccagno B, Giordano R, Pellegrino M, Broglio F, Gianotti L, Maccario M, Camanni F, Ghigo E (2001) Mineralocorticoid receptor blockade by canrenoate increases both spontaneous and stimulated adrenal function in humans. J Clin Endocrinol Metab 86(7):3176–3181
Grottoli S, Giordano R, Maccagno B, Pellegrino M, Ghigo E, Arvat E (2002) The stimulatory effect of canrenoate, a mineralocorticoid antagonist, on the activity of the hypothalamus-pituitary-adrenal axis is abolished by alprazolam, a benzodiazepine, in humans. J Clin Endocrinol Metab 87(10):4616–4620
Funder JW (1997) Glucocorticoid and mineralcorticoid receptors: biology and clinical relevance. Annu Rev Med 48:231–240
Ribeiro RC, Kushner PJ, Baxter JD (1995) The nuclear hormone receptor gene superfamily. Annu Rev Med 46:443–453
Grossmann C, Scholz T, Rochel M, Bumke-Vogt C, Oelkers W, Pfeiffer AF, Diederich S, Bahr V (2004) Transactivation via the human glucocorticoid and mineralocorticoid receptor by therapeutically used steroids in CV-1 cells: a comparison of their glucocorticoid and mineralocorticoid properties. Eur J Endocrinol 151(3):397–406
Reul JM, de Kloet ER (1985) Two receptor systems for corticosterone in rat brain: microdistribution and differential occupation. Endocrinology 117(6):2505–2511
Reul JM, Gesing A, Droste S, Stec IS, Weber A, Bachmann C, Bilang-Bleuel A, Holsboer F, Linthorst AC (2000) The brain mineralocorticoid receptor: greedy for ligand, mysterious in function. Eur J Pharmacol 405(1–3):235–249
Dallman MF, Levin N, Cascio CS, Akana SF, Jacobson L, Kuhn RW (1989) Pharmacological evidence that the inhibition of diurnal adrenocorticotropin secretion by corticosteroids is mediated via type I corticosterone-preferring receptors. Endocrinology 124(6):2844–2850
Spencer RL, Miller AH, Moday H, Stein M, McEwen BS (1993) Diurnal differences in basal and acute stress levels of type I and type II adrenal steroid receptor activation in neural and immune tissues. Endocrinology 133(5):1941–1950
Kalman BA, Spencer RL (2002) Rapid corticosteroid-dependent regulation of mineralocorticoid receptor protein expression in rat brain. Endocrinology 143(11):4184–4195
Otte C, Jahn H, Yassouridis A, Arlt J, Stober N, Maass P, Wiedemann K, Kellner M (2003) The mineralcorticoid receptor agonist, fludrocortisone, inhibits pituitary-adrenal activity in humans after pre-treatment with metyrapone. Life Sci 73(14):1835–1845
Otte C, Yassouridis A, Jahn H, Maass P, Stober N, Wiedemann K, Kellner M (2003) Mineralocorticoid receptor-mediated inhibition of the hypothalamic-pituitary-adrenal axis in aged humans. J Gerontol A Biol Sci Med Sci 58(10):900–905
Herman JP, Prewitt CM, Cullinan WE (1996) Neuronal circuit regulation of the hypothalamo-pituitary-adrenocortical stress axis. Crit Rev Neurobiol 10(3–4):371–394
Herman JP, Watson SJ, Spencer R (1999) Defense of adrenocorticosteroid receptor expression in rat hippocampus: effects of stress and strain. Endocrinology 140(9):3981–3991
Swanson LW, Sawchenko PE, Lind RW, Rho JH (1987) The CRH motoneuron: differential peptide regulation in neurons with possible synaptic, paracrine, and endocrine outputs. Ann NY Acad Sci 512:12–23
Swanson LW (1991) Biochemical switching in hypothalamic circuits mediating responses to stress. Prog Brain Res 87:181–200
Oitzl MS, Van Haarst AD, Sutanto W, De Kloet ER (1995) Corticosterone, brain mineralocorticoid receptor (MRs) and the activity of the hypothalamic-pituitary-adrenal (HPA) axis: the Lewis rat as an example of increased central MR capacity and a hyporesponsive HPA axis. Psychoneuroendocrinology 20(6):655–675
Ratka A, Sutanto W, Bloemers M, De Kloet ER (1989) On the role of brain mineralocorticoid (type I) and glucocorticoid (type II) receptors in neuroendocrine regulation. Neuroendocrinology 50(2):117–123
Deuschle M, Weber B, Colla M, Muller M, Kniest A, Heuser I (1998) Mineralocorticoid receptor also modulates basal activity of hypothalamus-pituitary-adrenocortical system in humans. Neuroendocrinology 68(5):355–360
Young EA, Lopez JF, Murphy-Weinberg V, Watson SJ, Akil H (1998) The role of mineralcorticoid receptors in hypothalamo-pituitary-adrenocortical axis regulation in humans. J Clin Endocrinol Metab 83(9):3339–3345
Born J, De Kloet ER, Wenz H, Kern W, Fehm HL (1991) Gluco- and antimineralocorticoid effects on human sleep: a role of central corticosteroid receptors. Am J Physiol 260:183–188
Wellhoener P, Born J, Fehm HL, Dodt C (2004) Elevated resting and exercise-induced cortisol levels after mineralocorticoid receptor blockade with canrenoate in healthy humans. J Clin Endocrinol Metab 89(10):5048–5052
Giordano R, Bo M, Pellegrino M, Vezzari M, Baldi M, Picu A, Balbo M, Bonelli L, Migliaretti G, Ghigo E, Arvat E (2005) Hypothalamus-pituitary-adrenal hyperactivity in human aging is partially refractory to stimulation by mineralcorticoid receptor blockade. J Clin Endocrinol Metab 90(10):5656–5662
Dodt C, Kern W, Fehm HL, Born J (1993) Antimineralocorticoid canrenoate enhances secretory activity of the hypothalamus-pituitary-adrenocortical (HPA) axis in humans. Neuroendocrinology 58(5):570–574
Funder JW, Carey RM, Fardella C, Gomez-Sanchez GE, Mantero F, Stowasser M, Young FW Jr, Montori VM (2008) Case detection, diagnosis, and treatment of patients with primary aldosteronism: an endocrine society clinical practice guideline. J Clin Endocrinol Metab 93:3266–3281
Berardelli R, Karamouzis I, Marinazzo E, Prats E, Picu A, Giordano R, Ghigo E, Arvat E (2010) Effect of acute and prolonged mineralocorticoid receptor blockade on spontaneous and stimulated hypothalamic-pituitary-adrenal axis in humans. Eur J Endocrinol 162(6):1067–1074
Giordano R, Pellegrino M, Picu A, Bonelli L, Oleandri SE, Pellissetto C, Limone P, Migliaretti G, Maccario M, Ghigo E, Arvat E (2007) Primary hyperaldosteronism is associated with derangement in the regulation of the hypothalamus-pituitary-adrenal axis in humans. J Endocrinol Invest 30(7):558–563
Heuser I, Deuschle M, Weber B, Stalla GK, Holsboer F (2000) Increased activity of the hypothalamus–pituitary–adrenal system after treatment with the mineralocorticoid receptor antagonist spironolactone. Psychoneuroendocrinology 25(5):513–518
Vogt W, Fischer I, Ebenroth S, Appel S, Knedel M, Lücker PW, Rennekamp H (1971) Pharmacokinetics of 9-fluorhydrocortisone. Arzneimittelforschung 21(8):1133–1143
Buckley TM, Mullen BC, Schatzberg AF (2007) The acute effects of a mineralocorticoid receptor (MR) agonist on nocturnal hypothalamic-adrenal-pituitary (HPA) axis activity in healthy controls. Psychoneuroendocrinology 32(8–10):859–864
Arvat E, Maccagno B, Ramunni J, Di Vito L, Giordano R, Gianotti L, Broglio F, Camanni F, Ghigo E (1999) The inhibitory effect of alprazolam, a benzodiazepine, overrides the stimulatory effect of metyrapone-induced lack of negative cortisol feedback on corticotroph secretion in humans. J Clin Endocrinol Metab 84:2611–2615
Grottoli S, Maccagno B, Ramunni J, Di Vito L, Giordano R, Gianotti L, Destefanis S, Camanni F, Ghigo E, Arvat E (2002) Alprazolam, a benzodiazepine, does not modify the ACTH and cortisol response to hCRH and AVP, but blunts the cortisol response to ACTH in humans. J Endocrinol Invest 25:420–425
Hügin-Flores ME, Steimer T, Aubert ML, Schulz P (2004) Mineralo- and glucocorticoid receptor mrnas are differently regulated by corticosterone in the rat hippocampus and anterior pituitary. Neuroendocrinology 79(4):174–184
Roubos EW, Kuribara M, Kuipers-Kwant FJ, Coenen TA, Meijer KH, Cruijsen PM, Denver RJ (2009) Dynamics of glucocorticoid and mineralocorticoid receptors in the Xenopus laevis pituitary pars intermedia. Ann NY Acad Sci 1163:292–295
Acknowledgments
This study was supported by Grants from the University of Turin and Foundation for the Study of Endocrine and Metabolic Diseases of Turin, Italy.
Conflict of interest
The authors declare that they have no conflict of interest.
Author information
Authors and Affiliations
Corresponding author
Additional information
Ioannis Karamouzis and Rita Berardelli equally contributed as first author.
Rights and permissions
About this article
Cite this article
Karamouzis, I., Berardelli, R., Marinazzo, E. et al. The acute effect of fludrocortisone on basal and hCRH-stimulated hypothalamic–pituitary–adrenal (HPA) axis in humans. Pituitary 16, 378–385 (2013). https://doi.org/10.1007/s11102-012-0435-3
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11102-012-0435-3