Abstract
Chronic fatigue syndrome (CFS) is a pathological state of extreme tiredness that lasts more than six months and may possess an impact on the social, emotional, or occupational functioning of an individual. CFS is characterized by profound disabling fatigue associated with infectious, rheumatological, and neurological symptoms. The current pharmacological treatment for CFS does not offer a complete cure for the disease, and none of the available treatments show promising results. The exact mechanism of the pathogenesis of the disease is still unknown, with current suggestions indicating the overlapping roles of the immune system, central nervous system, and neuroendocrine system. However, the pathological mechanism revolves around inflammatory and oxidative stress markers. Polyphenols are the most abundant secondary metabolites of plant origin, with potent antioxidant and anti-inflammatory effects, and can exert protective activity against a whole range of disorders. The current review is aimed at highlighting the emerging role of polyphenols in CFS from both preclinical and clinical studies. Numerous agents of this class have shown promising results in different in vitro and in vivo models of chronic fatigue/CFS, predominantly by counteracting oxidative stress and the inflammatory cascade. The clinical data in this regard is still very limited and needs expanding through randomized, placebo-controlled studies to draw final conclusions on whether polyphenols may be a class of clinically effective nutraceuticals in patients with CFS.
Graphical abstract

Similar content being viewed by others
Introduction
The word fatigue originates from the Latin verb “fatigare” which means ‘to tire’ and which can be defined as an extreme tiredness due to physical or mental exertion or illness (Phillips 2015; Kamal and Rahman 2018). It is a suboptimal psychophysiological state triggered by exertion, where the degree and dimension of the condition depends on the context and dynamics of exertion. The context of the exertion is explained by performance values for each individual, including circadian effects, sleep history, psychological factors, diet, individual traits, health fitness and other environmental conditions (Phillips 2015). Fatigue can be peripheral (physical) or central (mental), where physical fatigue refers to a reduction in individual capacity to perform physical tasks and mental fatigue refers to a reduction in individual capacity to perform tasks requiring concentration and alertness (Gawron et al. 2000). In healthy individuals, acute fatigue is a physiological reaction to prolonged and intense activity which is usually transient and does not interfere with daily activities and is generally reduced with rest (Kluger et al. 2013). The prolonged form of fatigue exists with same symptoms (acute symptoms) but may run for longer duration i.e., 30 days to 6 months (Esposito et al. 2022). Chronic fatigue syndrome (CFS) also known as Myalgic encephalomyelitis, occurs in diseased individuals, and is defined by an overwhelming sense of tiredness at rest and exhaustion after activities, interfering with daily tasks due to lack of energy (Walsh 2010). Chronic fatigue possesses an impact on the social, emotional, and occupational functioning of an individual, and may cause disability (Finsterer and Mahjoub 2014).
CFS is a pathological condition characterized by profound disabling fatigue lasting more than 6 months and is associated with infectious, rheumatological, and neurological symptoms. In addition to persistent fatigue (> 6 months) four or more of the following symptoms must occur to meet the criteria for CFS: aching or stiff muscles, tender glands, multi joint pain, sore throat, new headaches, impaired concentration, post-exertional fatigue, and unrefreshing sleep. Medical conditions that may explain prolonged fatigue exclude a patient from the diagnosis of CFS, as do a number of psychiatric illnesses such as melancholic depression, bipolar disorder, psychotic disorders, eating disorders, or substance use disorder within two years of the onset of fatigue (Afari and Buchwald 2003). Patients with CFS often present an abrupt onset of fatigue, flu-like illness, and exacerbation of fatigue with exertion. Many patients with CFS also experience nausea, anorexia, dizziness, drenching night sweats, an intolerance to pharmaceuticals (affecting the central nervous system) and alcohol (Afari and Buchwald 2003). The average prevalence of chronic fatigue in the adult population was estimated as 11.1% while the prevalence of CFS was estimated as 0.0004% in Australia to 3.6% in USA in the adult population. Moreover, the incidence and prevalence of CFS was 3 to 4-fold higher in women compared to men. The prevalence of chronic fatigue and CFS in Gulf war participants was 5.1% and 2.2%, respectively (Son 2012).
The clinical trials assessing the pharmacological options for the management of CFS are inconsistent and inconclusive. A systematic review on pharmacological treatments for CFS reported eleven medications to be mildly to moderately effective in their respective groups (Collatz et al. 2016). Six of them were found to provide significant results against fatigue, which included dextroamphetamine (Olson et al. 2003) and nefazodone (Hickie 1999), which were individually studied in clinical trials with inconclusive results. Intravenous immunoglobulin, acetyl-L-carnitine, and rintatolimod were other drugs reported in the systematic review, where each showed some improvements in severe symptoms, particularly against cognitive impairment and fatigue (Kerr et al. 2003; Malaguarnera et al. 2008; Strayer et al. 2012; Mitchell 2016). However, none of them showed promising results and none had the epidemiological significance to be considered gold standard in the pharmacological management of CFS.
The research on nutraceuticals has increased tremendously in recent decades due to increased demand, as they possess the potential to prevent or treat chronic disorders (Ullah et al. 2021). The nutraceutical market is expanding rapidly (though it is still in its initial phase) as the global nutraceutical market accounted for $379.061billion in 2017 and is expecting to expand by $734.601 billion in 2026 (Sachdeva et al. 2020; Ullah et al. 2021). Nutraceuticals can be classified into different groups based on food source i.e., polyphenols, spices, probiotics, prebiotics, dietary fibers, vitamins, and polyunsaturated fatty acids (Ullah et al. 2021). Numerous studies have shown promising results with nutraceuticals involved in the reduction of a number of disease risk factors including hyperglycemia, hypercholesterolemia, hypertension, altered mood conditions, and gastrointestinal discomfort (Fraga et al. 2019; Roudsari et al. 2019). Nutraceuticals and natural products have been shown to possess some sort of beneficial effect in the improvement of fatigue and associated symptoms, with a favorable safety profile (Luo et al. 2019), though the literature is still very much scarce in this regard. They may reduce oxidative stress and mitochondrial dysfunction, essential hallmarks of chronic fatigue, and thus they may be considered as alternative treatment agents for CFS (Simioni et al. 2018; Maksoud et al. 2021).
Polyphenols are secondary metabolites of plant origin. Chemically, they consist of benzene rings with OH moieties and range from the simpler ones like phenolic acids, to structurally complicated ones such as tannins (Khan et al. 2020a). Polyphenols can be classified into different groups including flavonoids, phenolic acids, stilbenes, flavonolignans, and curcuminoids (Daglia 2012; Durazzo et al. 2019; Ullah and Khan 2020). Flavonoid polyphenols are widespread in nature, and more than 11,000 flavonoids have been reported so far. Dietary sources of flavonoids include fruits, vegetables, tea, cocoa, and red wine, with a daily intake that ranges from 250 to more than 1000 mg. Based on oxidation levels and substitution patterns, they can be classified into flavones, flavanones, flavonols, flavan-3-ols, isoflavones, and anthocyanins (Khan et al. 2020b). A wide range of physiological properties has been reported following the intake of polyphenols in adequate amount such as antioxidant, anti-inflammatory, anti-diabetic, anti-obesity, cardioprotective, neuroprotective, anti-asthmatic, anxiolytic, antidepressant, antimicrobials, anticarcinogenic, to name a few (Ullah et al. 2020a, b; Zhao et al. 2020; Khan et al. 2021). While possessing capacity of several diseased targets and of multiple health benefits, polyphenols cover a huge share of global market of nutritional supplements. The estimated global market of polyphenols in 2018 was 1.28 billion US dollars, with a perspective increase expressed as compounded average growth rate (CAGR) of more than 7.2% from 2019 to 2025 (Abbasi-Parizad et al. 2022).
The aim of the current review is to focus on the pathogenic mechanisms of chronic fatigue and CFS and potential of polyphenols to fight against chronic fatigue, citing data from both preclinical and clinical studies. The present review consists of up-to-date literature review covering the basics of CFS and polyphenols as possible alternative agents. Various electronic databases were used for literature search including Google scholar, Scopus, Web of Science, and PubMed, using the keywords “fatigue” OR “chronic fatigue” OR “chronic fatigue syndrome” OR “Gulf War illness” OR “fibromyalgia” AND “polyphenols” OR “flavonoids” OR “resveratrol” OR “curcumin” AND “in vitro studies” OR “in vivo studies” OR “clinical studies” AND “safety aspects”. The criteria for selecting articles were “studies written in English because of language barrier”, and “preclinical and clinical studies reporting the beneficial properties of polyphenols in relation to chronic fatigue”. The results returned 205 papers published up to 2022. Of these articles, 155 were selected, summarized, and critically discussed to provide a comprehensive review. Some books and website links were also used for citing specific data within the scope of present study. Figure 1 illustrates the PRISMA flow diagram for study selection.
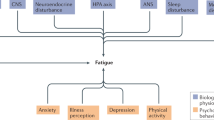
Biological pathogenesis of chronic fatigue
The exact pathogenesis of CFS remains elusive, however several mechanisms have been implicated in the alteration of immune function, hormonal regulation, metabolic changes, and host response to oxidative stress. Earlier theories have focused more on acute viral illnesses or psychiatric disorders while describing chronic fatigue. Many investigators have shown that CFS is a pathological condition with a complex and multifactorial etiology (Afari and Buchwald 2003). Figure 2 illustrates the schematic presentation of the pathogenic mechanisms of CFS.
Schematic diagram illustrating pathomechanisms involved in the pathology of CFS. Exogenous and endogenous stressors including viral and bacterial infections, exposure to chemicals, defects in immune system, hormonal imbalance and genetic defects can initiate the pathogenic mechanisms of CFS such as oxidative stress, low grade inflammation, altered immune response, mitochondrial dysfunction, low ATP levels, activation of neuroinflammatory pathways, and hypoperfusion of HPA axis. Natural killer cells (NK cells); regulatory T cells (Treg); cyclooxygenase-2 (COX-2); Inducible nitric oxide synthase (iNOS), nuclear factor kappa B (NF-κB); adenosine triphosphate (ATP); Hypothalamic–Pituitary–Adrenal axis (HPA axis)
Immune system
Zubieta et al. (1994) proposed that CFS is a persistent immune dysfunction initiated by certain infections such as viruses. The immune system hypothesis includes the alteration of the proper functioning of the central nervous system (CNS) associated with an abnormal response to common antigens, stimulation of the inflammatory and cell-mediated immune responses, and upstream regulation of oxidative and nitrosative pathways, and autoimmune responses against neuronal and other cells (Marshall-Gradisnik et al. 2016). A decreased response of T cells to antigens, downregulated functioning of natural killer (NK) cells, and altered cytokine profile have also been reported in numerous studies (Lorusso et al. 2009). The immunological findings defined CFS as a low-grade inflammatory disease, indicated by enhanced production of cyclooxygenase-2 (COX-2), inducible nitric oxide synthase (iNOS), and nuclear factor kappa B (NF-κB), dysregulated cytokine levels (increased levels of IL-1, IL-4, IL-5, IL-6, and IL-12 and decreased levels of IL-8, IL-13, and IL-15), autoimmune reactions, oxidative and nitrosative stress, reduced levels of antioxidant markers, mitochondrial dysfunction, increased translocation of Gram-negative bacteria, and altered antiviral response elements (i.e. 2–5 oligoadenylate synthetase/RNase L pathway) (Lorusso et al. 2009; Marshall-Gradisnik et al. 2016; Nguyen et al. 2017).
A viral infection may activate antiviral pathways, such as interferon gamma (IFN-γ)-indicated pathways, which could rapidly activate the establishment of a systemic inflammatory state (Maes et al. 2012). Enhanced reactive oxygen species (ROS) may alter the electron transport chain (ETC), resulting in the depletion of adenosine triphosphate (ATP) production which in turn leads to a deficiency of oxidative phosphorylation and impaired mitochondrial functioning (Kennedy et al. 2005; Maes 2009; Morris and Maes 2014). Viral-infections, oxidative stress and inflammatory cytokines can activate the NF-κB transcription factor, which could trigger an inflammatory cascade in patients. Once activated, NF-κB translocates from cytoplasm to nucleus where it binds to the DNA promoter sequences of TNF-α, IL-1β, IL-6, COX-2, and iNOS (Maes 2009; Tomas et al. 2017). This upregulation may link the interaction between increased oxidative stress and immunological overactivation. The actual directionality of this interaction is still not clear, however the immune response to an external pathogen could provoke unblinded oxidative stress which in turn perpetuates a steady inflammatory cascade (Maes et al. 2012; Rutherford et al. 2016).
The role of protein kinases in relation to immune system regulation is evident across the literature data. Impaired activity of mitogen-activated protein kinase (MAPK) could result in possible immunosuppression. This family of kinases is associated with the regulation of cell proliferation and cell death (Lu and Xu 2006). MAPK activation mediates proinflammatory signaling pathways i.e., increased production of proinflammatory cytokines (Sukkar et al. 2012). MAPK is required to initiate the p38/MAPK and the Janus kinase pathways (Lander et al. 1995; Park et al. 2000) and its inhibition (for instance by S-nitrosylation) may result in anti-inflammatory effects (Morris et al. 2017b). Tyrosine phosphorylation is a balanced activity of tyrosine kinases and tyrosine phosphatases, causing an increased production of proinflammatory mediators. Importantly, increased production of nitric oxide may activate tyrosine kinases and thus initiating and promoting the inflammatory damage in the body (Morris et al. 2017b). In addition, induction of apoptosis mediated by a dysregulated immune system and upstream regulation of inflammatory mediators has been observed by research studies. Vojdani et al. (1997) observed increased apoptotic cell population in CFS individuals along with enhanced protein kinase RNA (PKR) mRNA and protein levels. In same study, treatment with potent inhibitor of PKR 2-aminopurine resulted in the reduction of apoptotic population by more than 50%.
Central nervous system
The common symptoms of CFS, consisting of neuropsychiatric symptoms and chronic pain, suggest the alteration of CNS in the pathogenesis of the disease (Lange et al. 2004; Chen and Guilarte 2008; Nakatomi et al. 2014). Previous studies have shown hypoperfusion and reduction in the biosynthesis of neurotransmitters such as gamma aminobutyric acid (GABA), glutamate, and aspartate in the frontal, occipital, cingulate, and temporal cortices and basal ganglia. Some studies using single photon emission computed tomography (SPECT) demonstrated perfusion defects in the frontal and temporal lobes as well as impaired cerebral blood flow (Siemionow et al. 2004; Ji et al. 2013). A voxel-based morphometry study showed a volume reduction of the bilateral prefrontal cortices in patients with CFS, where volume reduction in the right prefrontal cortex has been associated with the severity of fatigue (Finkelmeyer et al. 2018). Neuroinflammation is actively involved in the pathogenesis of the disease, which is exhibited by the activation of glial cells, particularly astrocytes and microglia (Chen and Guilarte 2008; Nakatomi et al. 2014), resulting in an increased expression of 18-kDa translator protein, associated with the inflammatory activation of CNS (Chen and Guilarte 2008).
This constant interaction between pro-inflammatory cytokines and CNS leads to sickness, characterized by lassitude, fatigue, malaise, numbness, fewer social interactions, reduced appetite, and weight loss (Zhu et al. 2006; Dantzer 2009). In CFS patients, Hornig and colleagues demonstrated a considerable increase in pro-inflammatory cytokine levels in the cerebrospinal fluid, with immune activation and a shift towards T helper type-2 pattern associated with autoimmunity (Hornig et al. 2016). Later, Hornig and colleagues also suggested disturbed IL-1 signaling and autoimmunity-type patterns of immune activation while analyzing the cerebrospinal fluid in patients with atypical clinical characteristics (Hornig et al. 2017). Conversely, an upregulated inflammatory state cannot be countered by anti-inflammatory mechanisms, thus encouraging the development of the disease (Komaroff 2017). On one hand, the central inflammatory component may be triggered by the immunologic response to infectious agents (Glassford 2017). On other hand, patients with CFS exert significant effort to perform their daily activities, resulting in enhanced neural activation which may boost the production of pro-inflammatory cytokines and reactive oxygen and nitrogen species (Lange et al. 2004; Morris and Maes 2014; Schoeman et al. 2017).
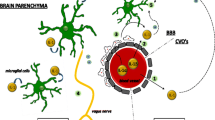
In addition to muscle fatigue, a central fatigue can be observed in CFS, characterized by a decline in the ability of muscle fibers to contract sufficiently during motor activity (Liu et al. 2005; Cotel et al. 2013). The cellular mechanism seems to be an increase in the levels of serotonin and its metabolites in CNS (Meyer et al. 2015; Yamashita and Yamamoto 2017). 5HT1A receptors are known to negatively regulate serotonin neurons and are expressed as presynaptic autoreceptors in the raphe nucleus and postsynaptic receptors on cortical, hippocampus, hypothalamic and spinal regions, implicated in emotion, mood, motor activity, and stress responses (Drevets et al. 2007; Cao and Li 2017). During high levels of release, serotonin spills over to reach extra-synaptic receptor sites in the initial axon segment of motoneurons, inhibiting action potential generation (Cotel et al. 2013). This phenomenon results in the prevention of hyperactivity in motoneurons, promoting motor unit rotation and thus reducing detrimental muscle activity (Liu et al. 2005). Numerous studies have suggested the potential role of inflammatory cytokines in the alteration of metabolism and release of neurotransmitters including serotonin. Pro-inflammatory cytokines i.e., TNF-α and IL-1β may activate the serotoninergic transporter (SERT) via the stimulation of the p38 MAPK pathway, increasing the concentration of other catecholamines in specific brain regions such as the anterior hypothalamus (Zhu et al. 2006; Farooq et al. 2017). SERT is associated with the transportation of serotonin into the presynaptic neurons from the synaptic cleft and along with 5-hydroxytryptamine transporter is involved in the termination of serotonergic signaling (Hensler 2010). Figure 3 illustrates the CNS signaling pathways associated with CFS pathogenesis.
Involvement of central nervous system in the pathogenesis of CFS. The peripheral immune response in subjects with CFS results in the activation of neuroinflammatory and other CNS associated signaling pathways. Initially microglial/astrocytes activation, increased expression of 18 kDa and excess release of serotonin leads to the stimulation of neuroinflammatory pathways such as Gateway reflux (entering of immune cells in CNS through “gateway” in the form of specific blood vessels), inflammatory reflux, loss of brain homeostasis, and BBB disruption, the ultimate result of which could be chronic neuroinflammation via microglial/astrocytes activation, IL-1 and TNF-α release, decreased monocytes and Th2 mediated autoimmunity. Chronic neuroinflammation may result in mitochondrial dysfunction which further aggravate the inflammatory cascade (via oxidative stress and altered purinergic signaling), leading to chronic serotonin release and HPA axis hypofunction, the ultimate result of which is cortisol dysfunction and, long and persistent CFS symptoms
Neuroendocrine system
The interest in the role the Hypothalamic–Pituitary–Adrenal (HPA) axis (Fig. 4) plays in the pathogenesis of CFS has been developed by clinical observations of low cortisol and debilitating fatigue (Morris et al. 2017a). Plenty of studies are available focusing on dysfunction of the HPA axis in patients with CFS, as hypoperfusion of the HPA axis is the most common biological finding in CFS (Torres-Harding et al. 2008; Tak et al. 2011). However, there is no convincing evidence that dysfunction of the HPA axis is specific to the disease or is the primary cause of the disorder, rather than being related to a number of possible consequences of the illness (Morris et al. 2017a). It has been hypothesized that low cortisol levels in these patients may be associated with the dysregulation of the stress response. Scott et al. suggested that CFS may be a stress related disorder and hypothesized that an initial stress may cause an increase in corticotropin release hormone (CRH) with a consequent downstream regulation of CRH receptors on pituitary corticotrophs neurons (Scott et al. 1998). On the contrary, this down-regulation of CRH receptors fails to normalize after mitigation of stress or reduction in CRH levels, perhaps due to abnormal plasticity of the CRH receptors. In short, stress induced HPA axis hyperfunction may switch into HPA axis hypofunction following prolonged stress (Rivera et al. 2019). Interestingly, chronic activation of the immune-inflammatory pathways could play a possible role in HPA axis hypofunction (Papadopoulos and Cleare 2012).
Neuroendocrine pathway and CFS pathogenesis. Stress induced HPA axis hyperfunction switches into HPA axis hypofunction following prolonged stress. Dysregulation of stress and chronic activation of immune inflammatory pathways may cause an increase in the release of CRF with a consequent down-regulation of CRF receptors on pituitary corticotropin neurons, and as a result HPA axis losses its ability to cope with environmental stress, coupled with decreased cortisol output. Corticotropin release hormone (CRF); adrenocorticotropic hormone (ACTH)
Mitochondrial dysfunction and insufficient ATP synthesis
Mitochondrial dysfunction is one of the most important pathologic hallmarks in CFS, characterized by suppressed mitochondrial respiration, impaired activities of ETC and mitochondrial membrane potential (MMP), and enhanced mitochondrial membrane permeability as a result of elevated levels of pro-inflammatory cytokines (TNF-α and IL-1) and increased oxidative and nitrosative stresses, the ultimate result of which could be altered ATP production and mitochondrial shutdown (Morris and Maes 2014). Activated oxidative and nitrosative pathways may also lead to damaging mitochondrial membranes and DNA, thus reducing membrane fluidity (Chen and Yu 1994). Additionally lowered levels of antioxidants such as zinc, omega-3 fatty acids, glutathione, and coenzyme Q10 in CFS may further aggravate the oxidative and nitrosative and immune-inflammatory pathways, worsening the condition of mitochondrial dysfunction and bioenergetic abnormalities (Maes et al. 2006, 2007, 2009; Shungu et al. 2012). Impaired ATP production and defects in ETC could be associated with increased mitochondrial production of superoxide and hydrogen peroxide radicals creating adaptive and synergistic damage. Literature data supported the potential role of mitochondrial dysfunctions (i.e., altered ATP production) in the onset of CFS symptoms such as fatigue and post exertional malaise (Morris and Maes 2014). As recently proposed, energy shutdown in primary biliary cholangitis is likely to occur by anti-pyruvate dehydrogenase complex autoantibodies. However, mitochondrial and metabolic dysfunction in CFS cannot be explained by the presence of circulating anti-mitochondrial autoantibodies (tested against the mitochondrial epitopes) (Nilsson et al. 2020).
With the reduction of the oxidative phosphorylation complex substrates (output of citric acid cycle) by glycolysis defect or pyruvate dehydrogenase deficiency may result in disturbances on cellular energy production (Armstrong et al. 2015; Fluge et al. 2016). Numerous studies have been reported a steady state reduction of ATP synthesis in patients with CFS (Myhill et al. 2009; Castro-Marrero et al. 2013; Brown et al. 2018). However, these steady-state measures do not define metabolic flux of the molecule of interest i.e., the rate of production and depletion. Real-time parameters of the cellular respiration or oxidative metabolism can be measured in the hepatic cells by extracellular flux assays by measuring oxygen consumption and extracellular acidification rates, and similar studies found no significant difference between absolute ATP synthesis rates between CFS and control cells (Tomas et al. 2017; Missailidis et al. 2020). However, this cannot exclude the possibility of ATP synthesis defect in patients with CFS as insufficient ATP synthesis could be offset by compensatory homeostatic mechanisms.
The homeostatic mechanism of cellular respiration is known to be regulated by two stress-sensing protein kinases i.e., target of rapamycin (TOR) (Ma and Blenis 2009) and AMP-activated protein kinase (AMPK) (Hardie and Carling 1997), often playing an inhibitory role. Metabolic abnormalities induced dysregulation of the activities of TOR and AMPK could result in their unresponsiveness to additional energy demand at cellular level. This has been supported by studies reporting insensitivity of AMPK in muscle cells to stimulation by contraction-induced ATP depletion (Brown et al. 2015, 2018). Such unresponsiveness may result either from AMPK already being in activated state in these cells or its inhibition by chronically hyperactivated TOR complex 1 (TORC1). Upregulated activity of TORC1 has been observed in lymphoblasts from CFS cells, associated with insufficient ATP synthesis and irregularly high expression of mitochondrial proteins (Missailidis et al. 2020). Moreover, reduced levels of creatinine kinase (CK) in the serum of patients with CFS may suggest lower cellular CK presence (responsible for cellular regulation of ATP levels) (Nacul et al. 2019), that may also contribute towards insufficient ATP synthesis (Wallimann et al. 1992).
Genetic basis
The possibility of diagnosing multiple members from same family with CFS implies the contribution of genetic factors to CFS risk (Walsh et al. 2001; Underhill and O’gorman 2006). However, CFS diagnosis do not follow a predictable Mendelian pattern, revealing that there is not one genetic variant that may increase CFS risk. Hence, CFS is multifactorial disease and may have many and varied genetic contributions (Li et al. 2015). Saiki and colleagues identified marker genes for the differential diagnosis of CFS i.e., the expression of GZMA, PSMA3, PSMA4, COX5B, ATP5J2, and DB1 was decreased while the expression of STAT5A was upregulated in CFS individuals (Saiki et al. 2008). GZMA encodes T cells and NK cells specific serine protease, functioning as a common component for the lysis of target cells (Lieberman 2010). The proteosome subunits PSMA3 and PSMA4, are components of central proteolytic system in addition to their role in processing of major histocompatibility complex-class I antigen and T cell activation (Saiki et al. 2008). COX5B and ATP5J2 are genes encoding molecules catalyzing oxidative phosphorylation in mitochondria (Vernon et al. 2002). These nuclear-encoded subunits regulate and assemble mitochondrial ATPase and cytochrome c oxidase complex. DBI (diazepam binding inhibitor also known as GABA receptor modulator or acyl-coenzyme A (acyl-CoA) binding protein (ACBP) acts as intracellular acyl-CoA transporter, forming a pool of ACBP-acyl-CoA complex which is an important intermediate in lipid metabolism (Alquier et al. 2021). In study by Saiki et al. (2008) the upregulated mRNA expression of COX5B, ATP5J2 and DBI genes strongly suggested the abnormalities energy metabolism in CSF subjects. STAT5A encoded protein, a member of STAT family of transcription factors. STAT5 regulates the signal transduction triggered by numerous cell ligands i.e., colony-stimulating factor 1, growth hormones, IL2, and IL4. Adult growth hormone deficiency is a CFS-like disease characterized by tiredness, fatigue, and myalgia, associated with impaired STAT5 signal transduction pathway (Webb et al. 2003). The downregulated STAT5A mRNA expression in CFS patients demonstrated the possible association of abnormal STAT5 signaling with the symptoms of CFS. Chacko et al. demonstrated the significant dysregulation of 92 protein kinase gene expression in NK cells in CFS patients, where 37 genes were upregulated, and 55 genes were downregulated (Chacko et al. 2016), potentially contributing to the pathomechanism of the disorder. Authors concluded that similar alterations in gene expression may also be present in other cells. A study also reported the enhanced gene expression of adrenergic receptor α1 (ADRA1A) in CFS samples as compared to healthy controls, assessed in peripheral blood mononuclear cells (Johnston et al. 2016). Besides smooth muscles contraction, ADRA1A also involved in gluconeogenesis and glycogenolysis of adipose tissues in the liver. Adrenergic receptor α1 stimulates Ca2+ influx via activation of phospholipase C followed by increase in intracellular concentration of inositol triphosphate (IP3) and diacylglycerol (DAG).
Associated diseased conditions
Some diseases may trigger the initiation and progression of CFS, the most common of which are chronic infections. In about 1/10th of individuals with Epstein-Barr virus, Ross River virus, or Coxiella burnetti are more prone to develop symptoms meeting criteria for CFS (also known as post-infective fatigue syndrome), associated mainly with the severity of the infection. Other infections including human herpesvirus 6, rubella, enterovirus, bornaviruses, Candida albicans, human immunodeficiency virus (HIV), and mycoplasma have been studied in connection to CFS but are not found to cause CFS (Centers for Disease Control and Prevention 2018). A logical explanation for the post-infective syndrome is the initiation of autoreactive process affecting numerous functions including brain and metabolism. These patients (mainly with genetic predisposition and/or dysbiosis) usually develop B cell clones that are prone to autoreactivity. Chronic infections may lead to immunization against autoantigens involving in aerobic energy production, ion channel proteins, and hormone proteins, thereby producing post-exertional malaise and fatigue, affecting both brain and muscle (Blomberg et al. 2018). Moreover, study showed a positive correlation of persistent fatigued symptoms with IL-2 production and negative correlation with IFN-γ/IL-2 ration in case of Q fever infections (bacterial infections causing flu-like symptoms) (Keijmel et al. 2016). Moreover, it is not uncommon that individuals with CFS-like illness often present with an overactive immune system, and thus significant number of CFS patients may have other immune-related or autoimmune disorders such as Hashimoto’s disease or fibromyalgia (Russell et al. 2019). These individuals have more frequently positive history of autoimmune diseases.
Diabetes (more commonly diabetes mellitus type 1) and related disorders (high sugar and low energy connection) also increase the risk for fatigue, muscle pain, exercise tolerance and delayed recovery. Post-exertional oxygen uptake significantly reduced, delayed and prolonged in diabetic patients, that may depend on impaired vascular/endothelial functioning in skeletal muscles (Bauer et al. 2007). Insulin resistance is associated with an increase in the levels of TNF-α, IL-6, and C-reactive protein (CRP) resulting in low grade inflammation (Tangvarasittichai et al. 2016). These inflammatory markers are also seen in CFS, suggesting that similar type of inflammation could be occurring in CFS In addition NK cells induced cytotoxicity also impaired in diabetes (Brauner et al. 2010) and impaired NK cell cytotoxicity is one of the most common hallmarks of inflammatory changes in CFS. Circulating leptin levels also play role in CFS symptomology, as chronically elevated leptin level is a maker of inflammation (Musker et al. 2021). Peripheral neuropathy frequently present in untreated patients with diabetes or other glucose dysregulating syndromes (i.e., impaired glucose tolerance and metabolic syndrome), sarcoidosis, thyroid dysfunction, vitamin B12 deficiency, and Gulf war illness have overlapping symptoms with CFS and fibromyalgia (Hovaguimian and Gibbons 2011; Li et al. 2014).
Polyphenols: nutraceutical approach towards chronic fatigue
Polyphenols are one of the most potent antioxidant phytochemicals, whose antioxidant effects are attributed to their ability to increase antioxidant defenses. This class of phytochemicals also exhibits anti-inflammatory activities via regulation of cellular activities in inflammatory cells and modulation of enzymatic activities involved in arachidonic acid metabolism (such as phospholipase A2 and COX) and arginine metabolism (such as NOS), as well as modulating the production of other proinflammatory molecules (Hussain et al. 2016). Recent research has discovered antifatigue capabilities exerted by polyphenols, as they alter free radical production and accumulation and thus slow down the rapid declining of muscular strength (Zhou and Jiang 2019). Besides, polyphenols also influence other mechanisms which tend towards improvement of muscle performance, for instance mitochondrial biogenesis comprises of two methods, either stimulating stress-related cell signaling pathways, increasing expression of genes encoding cytoprotective proteins such as nuclear respiratory factor 2 (NRF2) where variants of the NRF2 gene have been associated with endurance performance, or modulating muscle function and mitochondrial biogenesis by upstream regulation of sirtuins (SIRT1) and peroxisome proliferator-activated receptor γ coactivator (PGC-1α) activity (Somerville et al. 2017). Table 1 summarized some of the preclinical studies reported in the literature highlighting the effectiveness of polyphenols regards to CFS.
The antioxidant and antifatigue effects of Okra seeds and okra skin were determined by Xia and colleagues in male ICR mice (Xia et al. 2015). Okra seeds were found to possess significant antioxidant effects demonstrated by 1-diphenyl-2-picrylhydrazyl (DPPH) scavenging, ferric reducing antioxidant power (FRAP), and reducing power test. Moreover, they showed antifatigue effects in a weight-loaded swimming test. Biochemical determination showed a decrease in the levels of blood lactic acid and blood urea nitrogen (BUN), increased hepatic glycogen storage, reduced malondialdehyde (MDA) levels, and enhanced superoxide dismutase (SOD) and glutathione peroxidase levels. Okra skin did not show antifatigue effects. Interestingly, the phytochemical evaluation of the extracts revealed that the effects observed were largely attributable to the presence of flavonoids (isoquercitrin and quercetin-3-O-gentiobiose) in Okra seeds.
Apple polyphenols may enhance the proportions of fatigue-resistant myofibers and thus have been suggested as a possible nutraceutical for improving function in age-related muscle atrophy (Mizunoya et al. 2017). Supplementation of male Sprague Dawley rats with 0.5% apple polyphenols in an AIN-93G diet for 8-weeks significantly upregulated slower myosin-heavy-chain (MyHC) isoform ratios (IIx and IIa relative to total MyHC) and myoglobin expression in lower hind-limb muscles. An increase in fatigue resistance was observed, as recorded from measurements of relative plantar-flexion force torque generated by a stimulus train delivered to the tibial nerve. Earlier, Mizunoya et al. (2015) demonstrated positive effects of apple polyphenols on muscle endurance of young-adult rats. An intake of apple polyphenols (5% w/w in the diet) for 8 weeks upregulated the ratio of MyHC type IIx/IIb and myoglobin protein expression in plantaris muscles in male Fischer F344 rats, which indicated the application of apple polyphenols to reduce muscle decline due to aging and associated issues.
Prolivols, an olive fruit extract rich in polyphenols and hydroxytyrosol, was found to exert a potential role in combating CFS (Gupta et al. 2010). After lipopolysaccharide/Brucella abortus administration, the treatment of mice with olive extract (400 mg/kg p.o.) for 19 days significantly decreased immobility time and hyperalgesia along with an attenuation of oxidative stress (brain lipid peroxidation, reduced glutathione (GSH) levels, brain nitrite levels) and serum TNF-α levels.
Flavonoids, in combination with omega-3 fatty acids, ameliorated muscle and cardiac damage in a Duchenne muscular dystrophy (DMD) animal model (Tripodi et al. 2021). Dystrophic muscles are described by their low regenerative capacity, fiber necrosis, fibrosis, inflammatory processes, inadequate antioxidant response, and altered autophagic flux. Supplementation of mice with FLAVOmega β (a mixture of flavonoids and docosahexaenoic acid) ameliorated the pathological phenotype in dystrophic mice, as reflected by decreasing ROS production, suppressed inflammation and fibrosis, an oxidative metabolic switch of myofibrosis as well as increased mitochondrial activity, fatigue resistance, and vascularization. This study suggested the beneficial effect of FLAVOmega β supplements for preventing muscle weakness, delaying inflammatory processes, and sustaining physical health in DMD victims.
Isoflavones (found in soy and soy-based products) possess antioxidant and anti-inflammatory potential (Yu et al. 2016), and have been suggested to be useful in the treatment of CFS (Vasiadi et al. 2014). The treatment of mice with daidzein and genistein (low dose 20 mg/kg or high dose 150–200 mg/kg) for two weeks reversed the polyinosinic:polycytidylic acid [poly(I:C)]-induced decrease in locomotor activity and brain/skin gene expression, as well as serum levels of pro-inflammatory mediators relevant to CFS (i.e., TNF-α, IL-6, keratinocytes-derived chemokine (IL-8/(CXC motif) ligand (CXC)L8 murine homolog), chemokine (C–C motif) ligand-2, -4, -5 (CCL2, CCL4, CCL5) and C-X-C motif chemokine ligand 10 (CXCL10)).
The protective effect of quercetin on disused muscle atrophy was evaluated by Mukai et al. by targeting mitochondria in denervated mice (Mukai et al. 2016). A mouse denervation model was established via cutting the sciatic nerve in the right leg (SNX surgery) while pre-intake of a quercetin mixed diet for 14 days before denervation preserved muscle mass and atrophy of muscle fibers in the gastrocnemius muscle. A quercetin rich diet promoted the phosphorylation of Akt (a key phosphorylation pathway for the suppression of protein degradation) in mice with and without SNX surgery. Moreover, quercetin suppressed hydrogen peroxide generation (originating from mitochondria) along with the elevation of mitochondrial peroxisome proliferator-activated receptor-γ coactivator 1α (PGC-1α) mRNA expression (master regulator of mitochondrial biogenesis in the skeletal muscles) and NADH dehydrogenase-4 expression in the e gastrocnemius muscle in mice with SNX surgery.
Quercetin-3-O-gentiobiose, isolated from the tonic herb Okra, possessed antioxidant and anti-inflammatory activities resulting in the attenuation of chronic fatigue induced by endurance swimming in rats (Lin et al. 2014). In addition to vasoprotection, oral administration of quercetin-3-O-gentiobiose (25–75 mg/kg) once daily for two weeks considerably improved the endurance capability of rats against fatigue, decreased serum blood urea nitrogen (BUN) and lactic acid levels, enhanced the activities of antioxidant enzymes (SOD, glutathione peroxidase) and attenuated inflammatory cytokine levels (MCP-1, IL-6 and TNF-α). The study suggested a potential use for quercetin-3-O-gentiobiose for the treatment of aortic pathologies associated with chronic fatigue and related syndromes.
A dietary intake of rutin and curcumin (9.42 mg/animal/day) ameliorated muscle wasting induced by HPV16 in transgenic mice via NF-κB inhibition (Costa et al. 2017), suggesting that NF-κB inhibitors can be useful in treating cancer cachexia and chronic fatigue. Both compounds were orally administered to HPV16 transgenic mice (showing characteristic multi-step skin carcinogenesis) for 24-weeks. Both compounds totally suppressed mortality while decreasing white blood cell counts and the occurrence of splenitis and hepatitis. Rutin prevented muscle wastage more effectively than curcumin, where preservation of muscle mass was associated with downstream regulation of NF-κB canonical pathway.
Epigallocatechin gallate (EGCG) ameliorated fatigue values in a rodent model of CFS by reducing the lipopolysaccharide (LPS) induced oxidative and inflammatory mediators (Sachdeva et al. 2009; Sachdeva and Anurag Kuhad 2011). Treatment of rats with EGCG (5 and 10 mg/kg) for 28 days, 30 min before a forced swim session, restored all behavioral and biochemical parameters associated with CFS i.e., oxidative-nitrosative stress and serum TNF-α levels (Sachdeva and Anurag Kuhad 2011). Chronic treatment with EGCG (50 and 100 mg/kg, oral gavage 30 min before lipopolysaccharide/Brucella abortus administration) for 21 days restored the period of immobility, post swim fatigue, thermal hyperalgesia, oxidative–nitrosative stress (increased lipid peroxidation, nitrite levels and decreased endogenous antioxidant enzymes such as SOD, GSH and catalase), and inflammatory mediators (increased TNF-α and tissue growth factor-β (TGF-β) levels) in a mouse model of immunologically induced chronic fatigue (Sachdeva et al. 2009).
Green tea extract (25 or 50 mg/kg i.p.) and catechin (50 or 100 mg/kg i.p.), administered for 7 days, reversed the immobility time correlated with increased GSH and decreased lipid peroxidation in brains of fatigued mice (Singal et al. 2005). Similarly, green tea extract and its major polyphenol (i.e., EGCG) improved muscle function in a mouse model of DMD (Dorchies et al. 2006). A treatment of mdx5Cv mice with green tea extracts (0.05% or 0.25% weight/weight) and EGCG (0.1% weight/weight) for 1-week or 5-weeks resulted in delayed necrosis of the extensor digitorum longus muscle. Additionally, the phasic and tetanic tensions were enhanced, and the phasic-to-tonic tension ratio was corrected in the treated mice. Muscles of treated mice showed 30–50% more residual force in a fatigue assay. Recently, green tea extract and gallic acid showed antioxidant potential in murine models of post-stroke depression, evaluated by GSH, SOD, and thiobarbituric acid-reactive substance (TBARS) (Lorenzo et al. 2016; Nabavi et al. 2016). However, same results should be confirmed in research studies using specific experimental models of chronic fatigue.
From an experimental study using a mouse model of immunologically induced fatigue, Vij et al. (2009) suggested naringin as a possible alternative for the treatment of CFS. Mice challenged with lipopolysaccharide/Brucella abortus for 19 days displayed a considerable increase in immobility time, hyperalgesia, oxidative stress (brain lipoperoxidation, GSH and nitrite levels), and serum TNF-α levels. Concurrent treatment with naringin at different doses (i.e., 50, 100, and 200 mg/kg, p.o.) resulted in a significant decrease in immobility time, hyperalgesia, oxidative stress, and serum TNF-α levels, in a dose dependent manner.
Hesperetin (50 mg/kg/day), a flavanone glycoside found in citrus fruits restored the decrease in muscle fiber size in aged mice, treated for 8-weeks (Biesemann et al. 2018). In the same study hesperetin (10 µM) enhanced mitochondrial function by promoting ATP synthesis and mitochondrial share capacity in in vitro model of human differentiated myotubes, suggesting this agent as possible alternative for sarcopenia and retardation of mitochondrial dysfunction. Apigenin (25, 50, 100 mg/kg/day) intragastric administration for 9-months increased the muscle fiber size in aged mice, associated with the reduction of oxidative stress and inhibition of autophagy and apoptosis (Wang et al. 2020a). Apigenin also upregulated mitochondrial function by enhancing ATP levels and mitochondrial biogenesis via increasing expression of PGC-1α, Nrf-1, and ATP5B.
Anthocyanins may improve the regenerative capacity of skeletal muscle, possibly through redox control, enhancing protein synthesis and suppressing the breakdown of muscle protein, regulating mitochondrial function, inducing myogenesis, preventing apoptosis, regulating autophagy, and improving gut dysbiosis (Khairani et al. 2020).
Nutritional intervention with cyanidin delayed the progression of muscular dystrophy in dystrophic alpha-sarcoglyan (Sgca) null mice (Saclier et al. 2020). Sgca null mice were supplied with a cyanidin-enriched diet ad libitum for 5 or 25 weeks to evaluate the short-term and long-term benefits. Improved muscle morphology with fewer infiltrates was observed in the 5-week group, while the 25-week group retained muscle morphology with decreased necrosis and cellular infiltrates when compared to a cyanidin-free diet. A drastic reduction of extracellular matrix deposition was noted in mice fed with the cyanidin-rich diet, both at 5 and 25 weeks. No significant effects were found on the ratio of pro-inflammatory to anti-inflammatory macrophage subpopulations (CD64+-Ly6C+ and CD64+-Ly6C−, respectively) for the cyanidin-rich diet at both time points, suggesting that cyanidin may promote anti-inflammatory effects within the muscle environment (i.e., independent of macrophage phenotype). The quantification of F4/80+ macrophages revealed less macrophage infiltration in cyanidin supplemented Sgca mice. Moreover, cyanidin also upregulates antioxidant impact, reflected by a decrease in protein carbonylation, that may represent the synergistic outcome of enhanced oxidative metabolism and mitochondrial activity (Schiaffino and Reggiani 2011).
Murata et al. determined the preventive mechanisms of delphinidin on disused muscle atrophy using in vitro and in vivo experimental models and concluded that delphinidin may prevent muscle atrophy via upregulation of miR-23a and suppression of MuRF1 expression (Murata et al. 2017). Delphinidin (5 µM) suppressed dexamethasone induced MuRF1 expression in C2C12 cells, while the MuRF1 regulating pathway (forkhead box O [FoxO] transcription factors) remained unaffected. Delphinidin also reversed the downregulation of NFATc3 expression, and thus miR-23a expression in C2C12 cells challenged with dexamethasone. Oral administration of delphinidin (20 mg/kg/day) to male C57BL/6J mice resulted in the suppression of weight loss of the gastrocnemius and repression of MuRF1 expression along with the induction of NFATc3 and miR-23a expression in the gastrocnemius muscle. However, no effects were seen on quadriceps muscles.
To analyze the structural and/or functional abnormalities of the hippocampus in CFS, Moriya et al. established a CFS model by six repeated injections of Brucella abortus antigen to mice that manifested as reduced daily running activity and hippocampal atrophy (Moriya et al. 2011). The research team reported that resveratrol (a sirtuin-1 activator) may be an effective agent for improving fatigue symptoms and arresting hippocampal atrophy by suppressing apoptosis and boosting neurogenesis. An increase in daily running activity by 20% and an enlarged hippocampus were observed after the treatment with resveratrol (40 mg/kg p.o.) for 4-weeks. Resveratrol suppressed neuronal apoptosis and the expression of hippocampal acetylated p53, and improved neurogenesis and brain-derived neurotrophic factor mRNA in the hippocampus of the fatigued mice.
Gupta et al. (2009) also demonstrated the potential of curcumin to attenuate CFS in a murine water immersion stress model. Mice challenged with lipopolysaccharide/Brucella abortus for 19 days exhibited an increase in immobility time, hyperalgesia, and serum TNF-α levels. Concurrent treatment with curcumin (5, 30, 60 mg/kg, p.o.) for 19 days resulted in a significant decrease in immobility time, hyperalgesia, oxidative stress (brain lipid peroxidation, GSH levels and nitrite levels,) and TNF-α levels, in a dose dependent manner. Using experimental model of CKD-induced muscle atrophy in mice, Wang et al. (2020b) reported the amelioration of oxidative stress and mitochondrial dysfunction through suppressing GSK-3β activity with the supplementation of 0.04% dietary curcumin for 12-weeks. It enhanced mitochondrial biogenesis by increasing ATP synthesis and promoting the activity of mitochondrial electron transport chain. In another study, oral administration of curcumin (100 mg/kg/day) for 7 days protected purkinje neurons with the amelioration of motor function and reduction of cerebellar atrophy in rat model of cerebellar ataxia, challenged with neurotoxin 3-acetylpyridine (Mahmoudi et al. 2019). Curcumin improved motor coordination and muscular activity along with increased glutathione and decreased apoptotic marker cleaved caspase-3 levels.
Clinical trials
Although several studies using in vitro and in vivo experimental models have reported the protective effects of polyphenols in CFS and muscle atrophies, human use is still scarce in this area, perhaps due to ambiguities in polyphenol bioavailability, doses, and lack of efficacy in humans (Nikawa et al. 2021). Thus, clinical trials on polyphenols focusing on their potential use in clinical settings in patients with muscle fatigue and related syndrome need to be performed to determine their actual role in the improvement of fatigue. An exploratory multivariate study demonstrated a positive impact of polyphenols and caffeine intake on physical and mental fatigue (Boolani and Manierre 2019).
Similarly, consumption of grape and blueberry polyphenols and acute low/moderate doses of caffeine-free polyphenol rich coffeeberry extract improved cognitive performance and ameliorated mental fatigue in healthy adults during a sustained cognitive effort (Philip et al. 2019; Reed et al. 2019). Polyphenol-rich tart cherries (Prunus cerasus, cv Montmorency) improved mental fatigue along with sustained attention and feelings of alertness in middle-aged adults (Kimble et al. 2022). Table 2 summarized some of the clinical studies reported in the literature highlighting the clinical effectiveness of polyphenols in patients with CFS.
A randomized clinical trial (RCT) conducted by Sathyapalan et al. showed a reduction in the burden of symptoms of CFS with high cocoa polyphenol rich chocolate (Sathyapalan et al. 2010). The patients (mean age: 52 years) were treated either with an 8-week intervention of high cocoa liquor/polyphenol rich chocolate or simulated iso-calorific chocolate (cocoa liquor free/low polyphenols) with a wash-out period of 2-weeks followed by an 8-week intervention of other chocolate. Results showed significant improvement in Chalder Fatigue Scale scores, residual function (accessed by the London Handicap scale), and Hospital Anxiety and Depression scores after the 8-week intervention of high cocoa liquor/polyphenol rich chocolate. All the aforementioned parameters were significantly deteriorated with simulated iso-calorific chocolate. The polyphenols detected in high cocoa polyphenol rich chocolate were catechin, epicatechin, and procyanidins.
Supplementation of curcumin (in a phosphatidyl choline complex) for 8 weeks reduced CFS symptomatology, particularly in patients with milder cases (Campen and Visser 2019). Patients with ME/CFS (mean age: 47 years) were supplemented with curcumin (500 mg capsules twice a day) for 8 weeks. Fifty one out of sixty-five patients completed the trial, and result analysis demonstrated a significant decrease in CFS symptom scores defined by the center for disease control and prevention (CDC), and these effects were more pronounced in subjects with milder statuses of the disease.
Coe and colleagues, while evaluating the effects of flavonoid-rich cocoa for fatigue in relapsing and remitting multiple sclerosis (RRMS), concluded that a flavonoid rich beverage has the potential to improve fatigue and fatigability in patients with RRMS (Coe et al. 2019). 40 people with MS (10 men, 30 women; age 44 ± 10 years) were randomized to receive either high/low flavonoid cocoa beverage or placebo daily for 6-weeks. A mild effect of the intervention was seen on fatigue while a moderate effect was observed on fatigability.
Safety, tolerability, and efficacy of concord grape juice was established in Gulf war veterans with Gulf war illness by Helmer et al. in phase I/IIA randomized, double-blind, placebo-controlled trial (Helmer et al. 2020). Sixty Gulf war veterans with Gulf was illness (aged 42–65 years) were supplemented with concord grape juice (rich in flavonoids, anthocyanins, phenolic acids, trans-resveratrol) or placebo for 24-weeks. In the phase I dose-escalation portion, subjects were directed to consume 4-oz of grape juice or placebo daily for 2 weeks (weeks 0–2), 8-oz for 2 weeks (weeks 3–4), and finally 16-oz daily dose of either treatment (weeks 5–6). In the phase IIA steady-dose portion, patients were placed on the same steady dose (i.e., 16-oz daily for weeks 7–24). Thirty-one participants completed the study, and the results demonstrated a significant improvement in Halstead Category Test–Russell Revised Version (RCAT) scores. However, no significant differences were noted regards to cognitive functioning and fatigue variables in both treatment arms. Moreover, concord grape juice was found safe and well-tolerated across 24-weeks.
The efficacy and tolerability of a food supplement based on pomegranate extract, B vitamins, and vitamin C was successfully evaluated against prolonged fatigue in healthy subjects (Esposito et al. 2022). A preliminary evaluation of this food supplement involved 78-subjects (21 males and 57 females), aged 42.5 years for men and 39.8 years for women, that were supplemented with one stick pack of food supplement daily for one month (containing pomegranate extract 500 mg, ascorbic acid 200 mg, niacin 16 mg, riboflavin 7 mg, pantothenic acid 6 mg, thiamine 5.5 mg, pyridoxine 4 mg, folic acid 200 µg, biotin 50 µg, cobalamin 12.5 µg). The subjects reported a significant improvement in their condition, assessed by fatigue level and quality of life, without any adverse event being reported over this period of time. The chemical characterization of pomegranate extract showed a presence of ellagitannins, gallotannins, and organic and phenolic acids.
Safety concerns of polyphenols
Numerous in vitro and in vivo toxicological studies have shown that polyphenols are safe agents; however, the occurrence of toxicological effects remain a consideration, depending upon dose and duration of treatment (Nikawa et al. 2021). Catechin has been reported to damage DNA in mice spleen cells at higher concentrations (Fan and Lou 2004). Grape extracts (at a concentration range of 75–300 µg/mL) has been shown to promote mitomycin C, inducing sister chromatid exchange in human peripheral blood lymphocytes (Stagos et al. 2007). A mixture of caffeic acid, gallic acid, and rutin hydrate have also shown mitomycin C inducing clastogenicity at the same concentrations. Moreover, noticeable negative effects were seen with higher concentrations of epicatechin in fibroblast and keratinocyte cell lines when exposed for 24 h or more. Interestingly the compounds with gallate groups showed higher toxicity in comparison to those same compounds without gallate groups (Ugartondo et al. 2006). These studies indicate that polyphenols can be used safely when their intake is low. However, the dose of polyphenols alone is not a crucial factor when considering the toxic potential of phenolic compounds, as it also depends on the time of exposure and possible synergistic effects. Thus, it is essential to further extend the literature to investigate the safe doses and durations of treatment with polyphenols for safe and healthy applications (Fu et al. 2011).
Conclusions
Polyphenols is an interesting class of secondary metabolites with a range of biological properties, mainly attributed to their potent antioxidant and anti-inflammatory effects. Chronic fatigue has no effective treatment at the moment, and thus growing interest is focused on researching new agents for this condition. In recent years, the supplementation of antioxidants has greatly increased for the purpose of boosting muscular performance. In the present review, numerous in vitro and in vivo studies have shown that polyphenols from various classes (in particular flavonoids, resveratrol and curcumin) possess the potential to fight against CFS, and thus they can be used to develop effective therapeutic agents in this regard. However, the available literature is mainly based on preclinical studies and clinical studies are still in scarce to obtain some clear picture of the clinical effectiveness of polyphenols in the therapeutics of chronic fatigue. Thus, research should be expanded further to include studies on chronic fatigue states humans, before considering polyphenols for clinical use in patients with CFS. In particular, clinical research should be focused more on the antifatigue potential of individual polyphenols, their dosage and their duration of treatment. In addition, there are certain limitations to polyphenol usage in clinical settings mainly in term of their bioaccessibility and bioavailability concerns. A considerable decrease of total phenolic and total flavonoid contents has been observed in digested dietary samples (Cianciosi et al. 2020; Garzarella et al. 2022), and this issue is important to address while designing and performing clinical studies, to know the actual bioaccessible contents of phenolics and flavonoids available for absorption and pharmacological effects thereafter.
References
Abbasi-Parizad P, de Nisi P, Sciarria TP et al (2022) Polyphenol bioactivity evolution during the spontaneous fermentation of vegetal by-products. Food Chem 374:131791
Afari N, Buchwald D (2003) Chronic fatigue syndrome: a review. Am J Psychiatry 160:221–236
Alquier T, Christian-Hinman CA, Alfonso J, Færgeman NJ (2021) From benzodiazepines to fatty acids and beyond: revisiting the role of ACBP/DBI. Trends Endocrinol Metab 32:890–903
Armstrong CW, McGregor NR, Lewis DP et al (2015) Metabolic profiling reveals anomalous energy metabolism and oxidative stress pathways in chronic fatigue syndrome patients. Metabolomics 11:1626–1639
Bauer TA, Reusch JE, Levi M, Regensteiner JG (2007) Skeletal muscle deoxygenation after the onset of moderate exercise suggests slowed microvascular blood flow kinetics in type 2 diabetes. Diabetes Care 30:2880–2885
Biesemann N, Ried JS, Ding-Pfennigdorff D et al (2018) High throughput screening of mitochondrial bioenergetics in human differentiated myotubes identifies novel enhancers of muscle performance in aged mice. Sci Rep 8:9408
Blomberg J, Gottfries CG, Elfaitouri A et al (2018) Infection elicited autoimmunity and myalgic encephalomyelitis/chronic fatigue syndrome: an explanatory model. Front Immunol 9:229
Boolani A, Manierre M (2019) An exploratory multivariate study examining correlates of trait mental and physical fatigue and energy. Fatigue 7:29–40
Brauner H, Elemans M, Lemos S et al (2010) Distinct phenotype and function of NK cells in the pancreas of nonobese diabetic mice. J Immunol 184:2272–2280
Brown AE, Jones DE, Walker M, Newton JL (2015) Abnormalities of AMPK activation and glucose uptake in cultured skeletal muscle cells from individuals with chronic fatigue syndrome. PLoS ONE 10:e0122982
Brown AE, Dibnah B, Fisher E et al (2018) Pharmacological activation of AMPK and glucose uptake in cultured human skeletal muscle cells from patients with ME/CFS. Biosci Rep 38:BSR20180242
Cao Y, Li Q (2017) The variation of the 5-hydroxytryptamine system between chronic unpredictable mild stress rats and chronic fatigue syndrome rats induced by forced treadmill running. NeuroReport 28:630–637
Castro-Marrero J, Cordero MD, Sáez-Francas N et al (2013) Could mitochondrial dysfunction be a differentiating marker between chronic fatigue syndrome and fibromyalgia? Antioxid Redox Signal 19:1855–1860
Centers for Disease Control and Prevention (2018) Myalgic encephalomyelitis/chronic fatigue syndrome. https://www.cdc.gov/me-cfs/about/possible-causes.html. Accessed 26 Aug 2022
Chacko A, Staines DR, Johnston SC, Marshall-Gradisnik SM (2016) Dysregulation of protein kinase gene expression in NK cells from chronic fatigue syndrome/myalgic encephalomyelitis patients. Gene Regul Syst Biol 10:GRSB-S40036
Chen M-K, Guilarte TR (2008) Translocator protein 18 kDa (TSPO): molecular sensor of brain injury and repair. Pharmacol Ther 118:1–17
Chen JJ, Yu BP (1994) Alterations in mitochondrial membrane fluidity by lipid peroxidation products. Free Radic Biol Med 17:411–418
Cianciosi D, Forbes-Hernández TY, Giampieri F et al (2020) Effect of in vitro gastrointestinal digestion on the bioaccessibility of phenolic compounds and antioxidant activity of manuka honey. eFood 1:85–93
Coe S, Cossington J, Collett J et al (2019) A randomised double-blind placebo-controlled feasibility trial of flavonoid-rich cocoa for fatigue in people with relapsing and remitting multiple sclerosis. J Neurol Neurosurg Psychiatry 90:507–513
Collatz A, Johnston SC, Staines DR, Marshall-Gradisnik SM (2016) A systematic review of drug therapies for chronic fatigue syndrome/myalgic encephalomyelitis. Clin Ther 38:1263–1271
Cotel F, Exley R, Cragg SJ, Perrier J-F (2013) Serotonin spillover onto the axon initial segment of motoneurons induces central fatigue by inhibiting action potential initiation. Proc Natl Acad Sci USA 110:4774–4779
da Costa RMG, Aragão S, Moutinho M et al (2017) HPV16 induces a wasting syndrome in transgenic mice: amelioration by dietary polyphenols via NF-κB inhibition. Life Sci 169:11–19
Daglia M (2012) Polyphenols as antimicrobial agents. Curr Opin Biotechnol 23:174–181
Dantzer R (2009) Cytokine, sickness behavior, and depression. Immunol Allergy Clin N Am 29:247–264
de Lange FP, Kalkman JS, Bleijenberg G et al (2004) Neural correlates of the chronic fatigue syndrome—an fMRI study. Brain 127:1948–1957
di Lorenzo A, Nabavi SF, Sureda A et al (2016) Antidepressive-like effects and antioxidant activity of green tea and GABA green tea in a mouse model of post-stroke depression. Mol Nutr Food Res 60:566–579
Dorchies OM, Wagner S, Vuadens O et al (2006) Green tea extract and its major polyphenol (−)-epigallocatechin gallate improve muscle function in a mouse model for Duchenne muscular dystrophy. Am J Physiol Cell Physiol 290:C616–C625
Drevets WC, Thase ME, Moses-Kolko EL et al (2007) Serotonin-1A receptor imaging in recurrent depression: replication and literature review. Nucl Med Biol 34:865–877
Durazzo A, Lucarini M, Souto EB et al (2019) Polyphenols: a concise overview on the chemistry, occurrence, and human health. Phytother Res 33:2221–2243
Esposito C, Santarcangelo C, di Minno A et al (2022) Chemical characterization and preliminary evaluation of the efficacy and tolerability of a food supplement based on pomegranate extract, B vitamins, and vitamin C against prolonged fatigue in healthy consumers. Processes 10:208
Fan P, Lou H (2004) Effects of polyphenols from grape seeds on oxidative damage to cellular DNA. Mol Cell Biochem 267:67–74
Farooq RK, Asghar K, Kanwal S, Zulqernain A (2017) Role of inflammatory cytokines in depression: focus on interleukin-1β. Biomed Rep 6:15–20
Finkelmeyer A, He J, Maclachlan L et al (2018) Grey and white matter differences in chronic fatigue syndrome—a voxel-based morphometry study. NeuroImage Clin 17:24–30
Finsterer J, Mahjoub SZ (2014) Fatigue in healthy and diseased individuals. Am J Hosp Palliat Care 31:562–575
Fluge Ø, Mella O, Bruland O et al (2016) Metabolic profiling indicates impaired pyruvate dehydrogenase function in myalgic encephalopathy/chronic fatigue syndrome. JCI Insight 1:e89376
Fraga CG, Croft KD, Kennedy DO, Tomás-Barberán FA (2019) The effects of polyphenols and other bioactives on human health. Food Funct 10:514–528
Fu L, Xu B-T, Xu X-R et al (2011) Antioxidant capacities and total phenolic contents of 62 fruits. Food Chem 129:345–350
Garzarella EU, Navajas-Porras B, Pérez-Burillo S et al (2022) Evaluating the effects of a standardized polyphenol mixture extracted from poplar-type propolis on healthy and diseased human gut microbiota. Biomed Pharmacother 148:112759
Gawron VJ, French J, Funke D (2000) An overview of fatigue. Stress, workload, and fatigue. In: Hancock PA, Desmond PA (eds) Stress, workload, and fatigue. CRC Press, Boca Raton, pp 581–595
Glassford JAG (2017) The neuroinflammatory etiopathology of myalgic encephalomyelitis/chronic fatigue syndrome (ME/CFS). Front Physiol 8:1–9
Gupta A, Vij G, Sharma S et al (2009) Curcumin, a polyphenolic antioxidant, attenuates chronic fatigue syndrome in murine water immersion stress model. Immunobiology 214:33–39
Gupta A, Vij G, Chopra K (2010) Possible role of oxidative stress and immunological activation in mouse model of chronic fatigue syndrome and its attenuation by olive extract. J Neuroimmunol 226:3–7
Hardie DG, Carling D (1997) The AMP-activated protein kinase: fuel gauge of the mammalian cell? Eur J Biochem 246:259–273
Helmer DA, van Doren WW, Litke DR et al (2020) Safety, tolerability and efficacy of dietary supplementation with concord grape juice in Gulf war veterans with Gulf war illness: a phase I/IIA, randomized, double-blind, placebo-controlled trial. Int J Environ Res 17:3546
Hensler JG (2010) Serotonin in mood and emotion. In: Huston JP, Steiner H (eds) Handbook of behavioral neuroscience. Elsevier, Amsterdam, pp 367–378
Hickie I (1999) Nefazodone for patients with chronic fatigue syndrome. Aust N Z J Psychiatry 33:278–280
Hornig M, Gottschalk G, Peterson DL et al (2016) Cytokine network analysis of cerebrospinal fluid in myalgic encephalomyelitis/chronic fatigue syndrome. Mol Psychiatry 21:261–269
Hornig M, Gottschalk CG, Eddy ML et al (2017) Immune network analysis of cerebrospinal fluid in myalgic encephalomyelitis/chronic fatigue syndrome with atypical and classical presentations. Transl Psychiatry 7:e1080–e1080
Hovaguimian A, Gibbons CH (2011) Diagnosis and treatment of pain in small-fiber neuropathy. Curr Pain Headache Rep 15:193–200
Hussain T, Tan B, Yin Y et al (2016) Oxidative stress and inflammation: what polyphenols can do for us? Oxid Med Cell Longev 2016:7432797
Ji R-R, Berta T, Nedergaard M (2013) Glia and pain: is chronic pain a gliopathy? Pain 154:S10–S28
Johnston S, Staines D, Klein A, Marshall-Gradisnik S (2016) A targeted genome association study examining transient receptor potential ion channels, acetylcholine receptors, and adrenergic receptors in Chronic Fatigue Syndrome/Myalgic Encephalomyelitis. BMC Med Genet 17:79
Kamal M, Rahman MM (2018) Advances in fatigue life modeling: a review. Renew Sust Energ Rev 82:940–949
Keijmel SP, Raijmakers RP, Bleeker-Rovers CP et al (2016) Altered interferon-γ response in patients with Q-fever fatigue syndrome. J Infect 72:478–485
Kennedy G, Spence VA, McLaren M et al (2005) Oxidative stress levels are raised in chronic fatigue syndrome and are associated with clinical symptoms. Free Radic Biol Med 39:584–589
Kerr JR, Cunniffe VS, Kelleher P et al (2003) Successful intravenous immunoglobulin therapy in 3 cases of parvovirus B19-associated chronic fatigue syndrome. Clin Infect Dis 36:e100–e106
Khairani AF, Setiawan CJ, Shanty N et al (2020) Molecular mechanisms of anthocyanins as a potential nutraceutical for muscle regeneration. Sys Rev Pharm 11:189–202
Khan H, Reale M, Ullah H et al (2020a) Anti-cancer effects of polyphenols via targeting p53 signaling pathway: updates and future directions. Biotechnol Adv 38:107385
Khan H, Ullah H, Tundis R et al (2020b) Dietary flavonoids in the management of huntington’s disease: mechanism and clinical perspective. eFood 1:38–52
Khan H, Labanca F, Ullah H, et al (2021) Advances and challenges in cancer treatment and nutraceutical prevention: the possible role of dietary phenols in BRCA regulation. Phytochem Rev 1–16
Kimble R, Keane KM, Lodge JK, et al (2022) Polyphenol-rich tart cherries (Prunus cerasus, cv Montmorency) improve sustained attention, feelings of alertness and mental fatigue and influence the plasma metabolome in middle-aged adults: a randomised, placebo-controlled trial. Br J Nutr 1–31
Kluger BM, Krupp LB, Enoka RM (2013) Fatigue and fatigability in neurologic illnesses: proposal for a unified taxonomy. Neurology 80:409–416
Komaroff AL (2017) Inflammation correlates with symptoms in chronic fatigue syndrome. Proc Natl Acad Sci USA 114:8914–8916
Lander HM, Ogiste JS, Pearce SFA et al (1995) Nitric oxide-stimulated guanine nucleotide exchange on p21ras. J Biol Chem 270:7017–7020
Li M, Xu C, Yao W et al (2014) Self-reported post-exertional fatigue in Gulf War veterans: roles of autonomic testing. Front Neurosci 7:269
Li YR, Li J, Zhao SD et al (2015) Meta-analysis of shared genetic architecture across ten pediatric autoimmune diseases. Nat Med 21:1018–1027
Lieberman J (2010) Granzyme A activates another way to die. Immunol Rev 235:93–104
Lin Y, Liu H-L, Fang J et al (2014) Anti-fatigue and vasoprotective effects of quercetin-3-O-gentiobiose on oxidative stress and vascular endothelial dysfunction induced by endurance swimming in rats. Food Chem Toxicol 68:290–296
Liu JZ, Yao B, Siemionow V et al (2005) Fatigue induces greater brain signal reduction during sustained than preparation phase of maximal voluntary contraction. Brain Res 1057:113–126
Lorusso L, Mikhaylova SV, Capelli E et al (2009) Immunological aspects of chronic fatigue syndrome. Autoimmun Rev 8:287–291
Lu Z, Xu S (2006) ERK1/2 MAP kinases in cell survival and apoptosis. IUBMB Life 58:621–631
Luo C, Xu X, Wei X et al (2019) Natural medicines for the treatment of fatigue: bioactive components, pharmacology, and mechanisms. Pharmacol Res 148:104409
Ma XM, Blenis J (2009) Molecular mechanisms of mTOR-mediated translational control. Nat Rev Mol Cell Biol 10:307–318
Maes M (2009) Inflammatory and oxidative and nitrosative stress pathways underpinning chronic fatigue, somatization and psychosomatic symptoms. Curr Opin Psychiatry 22:75–83
Maes M, Mihaylova I, Leunis JC (2006) Chronic fatigue syndrome is accompanied by an IgM-related immune response directed against neopitopes formed by oxidative or nitrosative damage to lipids and proteins. Neuro Endocrinol Lett 27:615–622
Maes M, Mihaylova I, Leunis JC (2007) Increased serum IgM antibodies directed against phosphatidyl inositol (Pi) in chronic fatigue syndrome (CFS) and major depression: evidence that an IgM-mediated immune response against Pi is one factor underpinning the comorbidity between both CFS and dep. Neuro Endocrinol Lett 28:861–867
Maes M, Mihaylova I, Kubera M et al (2009) Coenzyme Q10 deficiency in myalgic encephalomyelitis/chronic fatigue syndrome (ME/CFS) is related to fatigue, autonomic and neurocognitive symptoms and is another risk factor explaining the early mortality in ME/CFS due to cardiovascular disorder. Neuro Endocrinol Lett 30:470–476
Maes M, Twisk FNM, Kubera M, Ringel K (2012) Evidence for inflammation and activation of cell-mediated immunity in myalgic encephalomyelitis/chronic fatigue syndrome (ME/CFS): increased interleukin-1, tumor necrosis factor-α, PMN-elastase, lysozyme and neopterin. J Affect Disord 136:933–939
Mahmoudi M, Bayat AH, Boroujeni ME et al (2019) Curcumin protects purkinje neurons, ameliorates motor function and reduces cerebellar atrophy in rat model of cerebellar ataxia induced by 3-AP. J Chem Neuroanat 102:101706
Maksoud R, Balinas C, Holden S et al (2021) A systematic review of nutraceutical interventions for mitochondrial dysfunctions in myalgic encephalomyelitis/chronic fatigue syndrome. J Transl Med 19:1–11
Malaguarnera M, Gargante MP, Cristaldi E et al (2008) Acetyl L-carnitine (ALC) treatment in elderly patients with fatigue. Arch Gerontol Geriatr 46:181–190
Marshall-Gradisnik S, Huth T, Chacko A et al (2016) Natural killer cells and single nucleotide polymorphisms of specific ion channels and receptor genes in myalgic encephalomyelitis/chronic fatigue syndrome. Appl Clin Genet 9:39
Meyer B, Nguyen CBT, Moen A et al (2015) Maintenance of chronic fatigue syndrome (CFS) in Young CFS patients is associated with the 5-HTTLPR and SNP rs25531 A> G Genotype. PLoS ONE 10:e0140883
Missailidis D, Annesley SJ, Allan CY et al (2020) An isolated complex V inefficiency and dysregulated mitochondrial function in immortalized lymphocytes from ME/CFS patients. Int J Mol Sci 21:1074
Mitchell WM (2016) Efficacy of rintatolimod in the treatment of chronic fatigue syndrome/myalgic encephalomyelitis (CFS/ME). Expert Rev Clin Pharmacol 9:755–770
Mizunoya W, Miyahara H, Okamoto S et al (2015) Improvement of endurance based on muscle fiber-type composition by treatment with dietary apple polyphenols in rats. PLoS ONE 10:e0134303
Mizunoya W, Okamoto S, Miyahara H et al (2017) Fast-to-slow shift of muscle fiber-type composition by dietary apple polyphenols in rats: impact of the low-dose supplementation. Anim Sci 88:489–499
Moriya J, Chen R, Yamakawa J-I et al (2011) Resveratrol improves hippocampal atrophy in chronic fatigue mice by enhancing neurogenesis and inhibiting apoptosis of granular cells. Biol Pharm Bull 34:354–359
Morris G, Maes M (2014) Mitochondrial dysfunctions in myalgic encephalomyelitis/chronic fatigue syndrome explained by activated immuno-inflammatory, oxidative and nitrosative stress pathways. Metab Brain Dis 29:19–36
Morris G, Anderson G, Maes M (2017a) Hypothalamic-pituitary-adrenal hypofunction in myalgic encephalomyelitis (ME)/chronic fatigue syndrome (CFS) as a consequence of activated immune-inflammatory and oxidative and nitrosative pathways. Mol Neurobiol 54:6806–6819
Morris G, Berk M, Klein H et al (2017b) Nitrosative stress, hypernitrosylation, and autoimmune responses to nitrosylated proteins: new pathways in neuroprogressive disorders including depression and chronic fatigue syndrome. Mol Neurobiol 54:4271–4291
Mukai R, Matsui N, Fujikura Y et al (2016) Preventive effect of dietary quercetin on disuse muscle atrophy by targeting mitochondria in denervated mice. J Nutr Biochem 31:67–76
Murata M, Nonaka H, Komatsu S et al (2017) Delphinidin prevents muscle atrophy and upregulates miR-23a expression. J Agric Food Chem 65:45–50
Musker M, McArthur A, Munn Z, Wong ML (2021) Circulating leptin levels in patients with myalgic encephalomyelitis, chronic fatigue syndrome or fibromyalgia: a systematic review protocol. JBI Evid Synth 19:695–701
Myhill S, Booth NE, McLaren-Howard J (2009) Chronic fatigue syndrome and mitochondrial dysfunction. Int J Clin Exp Med 2:1–16
Nabavi SF, Habtemariam S, di Lorenzo A et al (2016) Post-stroke depression modulation and in vivo antioxidant activity of gallic acid and its synthetic derivatives in a murine model system. Nutrients 8:248
Nacul L, de Barros B, Kingdon CC et al (2019) Evidence of clinical pathology abnormalities in people with myalgic encephalomyelitis/chronic fatigue syndrome (ME/CFS) from an analytic cross-sectional study. Diagnostics 9:41
Nakatomi Y, Mizuno K, Ishii A et al (2014) Neuroinflammation in patients with chronic fatigue syndrome/myalgic encephalomyelitis: an 11C-(R)-PK11195 PET study. J Nucl Med 55:945–950
Nguyen T, Johnston S, Clarke L et al (2017) Impaired calcium mobilization in natural killer cells from chronic fatigue syndrome/myalgic encephalomyelitis patients is associated with transient receptor potential melastatin 3 ion channels. Clin Exp Immunol 187:284–293
Nikawa T, Ulla A, Sakakibara I (2021) Polyphenols and their effects on muscle atrophy and muscle health. Molecules 26:4887
Nilsson I, Palmer J, Apostolou E et al (2020) Metabolic dysfunction in myalgic encephalomyelitis/chronic fatigue syndrome not due to anti-mitochondrial antibodies. Front Med 7:108
Olson LG, Ambrogetti A, Sutherland DC (2003) A pilot randomized controlled trial of dexamphetamine in patients with chronic fatigue syndrome. Psychosomatics 44:38–43
Papadopoulos AS, Cleare AJ (2012) Hypothalamic–pituitary–adrenal axis dysfunction in chronic fatigue syndrome. Nat Rev Endocrinol 8:22–32
Park HS, Huh SH, Kim MS et al (2000) Nitric oxide negatively regulates c-Jun N-terminal kinase/stress-activated protein kinase by means of S-nitrosylation. Proc Natl Acad Sci USA 97:14382–14387
Philip P, Sagaspe P, Taillard J et al (2019) Acute intake of a grape and blueberry polyphenol-rich extract ameliorates cognitive performance in healthy young adults during a sustained cognitive effort. Antioxidants 8:650
Phillips RO (2015) A review of definitions of fatigue—and a step towards a whole definition. Transp Res F Traffic Psychol Behav 29:48–56
Reed RA, Mitchell ES, Saunders C, O’Connor PJ (2019) Acute low and moderate doses of a caffeine-free polyphenol-rich coffeeberry extract improve feelings of alertness and fatigue resulting from the performance of fatiguing cognitive tasks. J Cogn Enhanc 3:193–206
Rivera MC, Mastronardi C, Silva-Aldana CT et al (2019) Myalgic encephalomyelitis/chronic fatigue syndrome: a comprehensive review. Diagnostics 9:91
Roudsari NM, Lashgari N-A, Momtaz S et al (2019) Natural polyphenols for the prevention of irritable bowel syndrome: molecular mechanisms and targets; a comprehensive review. DARU J Pharm Sci 27:755–780
Russell A, Hepgul N, Nikkheslat N et al (2019) Persistent fatigue induced by interferon-alpha: a novel, inflammation-based, proxy model of chronic fatigue syndrome. Psychoneuroendocrinology 100:276–285
Rutherford G, Manning P, Newton JL (2016) Understanding muscle dysfunction in chronic fatigue syndrome. J Aging Res 2016:1–13
Sachdeva AK, Anurag Kuhad KC (2011) Epigallocatechin gallate ameliorates behavioral and biochemical deficits in rat model of load-induced chronic fatigue syndrome. Brain Res Bull 86:165–172
Sachdeva AK, Kuhad A, Tiwari V, Chopra K (2009) Epigallocatechin gallate ameliorates chronic fatigue syndrome in mice: behavioral and biochemical evidence. Behav Brain Res 205:414–420
Sachdeva V, Roy A, Bharadvaja N (2020) Current prospects of nutraceuticals: a review. Curr Pharm Biotechnol 21:884–896
Saclier M, Bonfanti C, Antonini S et al (2020) Nutritional intervention with cyanidin hinders the progression of muscular dystrophy. Cell Death Dis 11:1–12
Saiki T, Kawai T, Morita K et al (2008) Identification of marker genes for differential diagnosis of chronic fatigue syndrome. Mol Med 14:599–607
Sathyapalan T, Beckett S, Rigby AS et al (2010) High cocoa polyphenol rich chocolate may reduce the burden of the symptoms in chronic fatigue syndrome. Nutr J 9:1–5
Schiaffino S, Reggiani C (2011) Fiber types in mammalian skeletal muscles. Physiol Rev 91:1447–1531
Schoeman EM, van der Westhuizen FH, Erasmus E et al (2017) Clinically proven mtDNA mutations are not common in those with chronic fatigue syndrome. BMC Med Genet 18:1–4
Scott LV, Burnett F, Medbak S, Dinan TG (1998) Naloxone-mediated activation of the hypothalamic–pituitary–adrenal axis in chronic fatigue syndrome. Psychol Med 28:285–293
Shungu DC, Weiduschat N, Murrough JW et al (2012) Increased ventricular lactate in chronic fatigue syndrome. III. Relationships to cortical glutathione and clinical symptoms implicate oxidative stress in disorder pathophysiology. NMR Biomed 25:1073–1087
Siemionow V, Fang Y, Calabrese L et al (2004) Altered central nervous system signal during motor performance in chronic fatigue syndrome. Clin Neurophysiol 115:2372–2381
Simioni C, Zauli G, Martelli AM et al (2018) Oxidative stress: role of physical exercise and antioxidant nutraceuticals in adulthood and aging. Oncotarget 9:17181
Singal A, Kaur S, Tirkey N, Chopra K (2005) Green tea extract and catechin ameliorate chronic fatigue-induced oxidative stress in mice. J Med Food 8:47–52
Somerville V, Bringans C, Braakhuis A (2017) Polyphenols and performance: a systematic review and meta-analysis. Sports Med 47:1589–1599
Son C-G (2012) Review of the prevalence of chronic fatigue worldwide. J Korean Med 33:25–33
Stagos D, Spanou C, Margariti M et al (2007) Cytogenetic effects of grape extracts (Vitis vinifera) and polyphenols on mitomycin C-induced sister chromatid exchanges (SCEs) in human blood lymphocytes. J Agric Food Chem 55:5246–5252
Strayer DR, Carter WA, Stouch BC et al (2012) A double-blind, placebo-controlled, randomized, clinical trial of the TLR-3 agonist rintatolimod in severe cases of chronic fatigue syndrome. PLoS ONE 7:e31334
Sukkar MB, Ammit AJ, Manetsch M et al (2012) TLR2 ligand engagement upregulates airway smooth. Am J Physiol Lung Cell Mol Physiol 302:L838–L845
Tak LM, Cleare AJ, Ormel J et al (2011) Meta-analysis and meta-regression of hypothalamic-pituitary-adrenal axis activity in functional somatic disorders. Biol Psychol 87:183–194
Tangvarasittichai S, Pongthaisong S, Tangvarasittichai O (2016) Tumor necrosis factor-Α, interleukin-6, C-reactive protein levels and insulin resistance associated with type 2 diabetes in abdominal obesity women. Indian J Clin Biochem 31:68–74
Tomas C, Brown A, Strassheim V et al (2017) Cellular bioenergetics is impaired in patients with chronic fatigue syndrome. PLoS ONE 12:e0186802
Torres-Harding S, Sorenson M, Jason L et al (2008) The associations between basal salivary cortisol and illness symptomatology in chronic fatigue syndrome. J Appl Biobehav Res 13:157–180
Tripodi L, Molinaro D, Farini A et al (2021) Flavonoids and omega3 prevent muscle and cardiac damage in Duchenne muscular dystrophy animal model. Cells 10:2917
Ugartondo V, Mitjans M, Lozano C et al (2006) Comparative study of the cytotoxicity induced by antioxidant epicatechin conjugates obtained from grape. J Agric Food Chem 54:6945–6950
Ullah H, Khan H (2020) Epigenetic drug development for autoimmune and inflammatory diseases. In: Castelo-Branco P, Jeronimo C (eds) Histone modifications in therapy, 1st edn. Academic Press, Cambridge, pp 395–413
Ullah H, de Filippis A, Khan H et al (2020a) An overview of the health benefits of Prunus species with special reference to metabolic syndrome risk factors. Food Chem Toxicol 144:111574
Ullah H, de Filippis A, Santarcangelo C, Daglia M (2020b) Epigenetic regulation by polyphenols in diabetes and related complications. Med J Nutr Metab 13:289–310
Ullah H, Tovchiga O, Daglia M, Khan H (2021) Modulating gut microbiota: an emerging approach in the prevention and treatment of multiple sclerosis. Curr Neuropharmacol 19:1966–1983
Underhill RA, O’gorman R, (2006) Prevalence of chronic fatigue syndrome and chronic fatigue within families of CFS patients. J Chronic Fatigue Syndr 13:3–13
van Campen CM, Visser FC (2019) The effect of curcumin in patients with chronic fatigue syndrome/myalgic encephalomyelitis disparate responses in different disease severities. Pharmacovigil Pharmacoepidemiol 2:22–27
Vasiadi M, Newman J, Theoharides TC (2014) Isoflavones inhibit poly (I: C)-induced serum, brain, and skin inflammatory mediators-relevance to chronic fatigue syndrome. J Neuroinflammation 11:1–12
Vernon SD, Unger ER, Dimulescu IM et al (2002) Utility of the blood for gene expression profiling and biomarker discovery in chronic fatigue syndrome. Dis Mark 18:193–199
Vij G, Gupta A, Chopra K (2009) Modulation of antigen-induced chronic fatigue in mouse model of water immersion stress by naringin, a polyphenolic antioxidant. Fundam Clin Pharmacol 23:331–337
Vojdani A, Ghoneum M, Choppa PC et al (1997) Elevated apoptotic cell population in patients with chronic fatigue syndrome: the pivotal role of protein kinase RNA. J Intern Med 242:465–478
Wallimann T, Wyss M, Brdiczka D et al (1992) Intracellular compartmentation, structure and function of creatine kinase isoenzymes in tissues with high and fluctuating energy demands: the’phosphocreatine circuit’for cellular energy homeostasis. Biochem J 281:21–40
Walsh D (2010) Mechanisms of fatigue. J Support Oncol 8:164–174
Walsh CM, Zainal NZ, Middleton SJ, Paykel ES (2001) A family history study of chronic fatigue syndrome. Psychiatr Genet 11:123–128
Wang D, Yang Y, Zou X et al (2020a) Antioxidant apigenin relieves age-related muscle atrophy by inhibiting oxidative stress and hyperactive mitophagy and apoptosis in skeletal muscle of mice. J Gerontol Ser A 75:2081–2088
Wang D, Yang Y, Zou X et al (2020b) Curcumin ameliorates CKD-induced mitochondrial dysfunction and oxidative stress through inhibiting GSK-3β activity. J Nutr Biochem 83:108404
Webb SM, de Andrés-Aguayo I, Rojas-García R et al (2003) Neuromuscular dysfunction in adult growth hormone deficiency. Clin Endocrinol 59:450–458
Xia F, Zhong Y, Li M et al (2015) Antioxidant and anti-fatigue constituents of Okra. Nutrients 7:8846–8858
Yamashita M, Yamamoto T (2017) Tryptophan circuit in fatigue: from blood to brain and cognition. Brain Res 1675:116–126
Yu J, Bi X, Yu B, Chen D (2016) Isoflavones: anti-inflammatory benefit and possible caveats. Nutrients 8:361
Zhao C, Wan X, Zhou S, Cao H (2020) Natural polyphenols: a potential therapeutic approach to hypoglycemia. eFood 1:107–118
Zhou S-S, Jiang J-G (2019) Anti-fatigue effects of active ingredients from traditional Chinese medicine: a review. Curr Med Chem 26:1833–1848
Zhu C-B, Blakely RD, Hewlett WA (2006) The proinflammatory cytokines interleukin-1beta and tumor necrosis factor-alpha activate serotonin transporters. Neuropsychopharmacology 31:2121–2131
Zubieta JK, Engleberg NC, Yargiç LI et al (1994) Seasonal symptom variation in patients with chronic fatigue: comparison with major mood disorders. J Psychiatr Res 28:13–22
Funding
Open access funding provided by Università degli Studi di Napoli Federico II within the CRUI-CARE Agreement.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
The authors declare that they have no conflict of interest.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/.
About this article
Cite this article
Ullah, H., Khan, A., Riccioni, C. et al. Polyphenols as possible alternative agents in chronic fatigue: a review. Phytochem Rev 22, 1637–1661 (2023). https://doi.org/10.1007/s11101-022-09838-9
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11101-022-09838-9