Abstract
Background
Colorectal cancer is a significant health concern worldwide, with metastatic CRC (mCRC) presenting a particularly challenging prognosis. The FRESCO-2 trial highlighted the potential of fruquintinib in heavily pretreated mCRC patients.
Aim
Given the recent changes in drug pricing in China and the evolving mCRC treatments, this study aimed to evaluate the cost-effectiveness of fruquintinib in the context of current Chinese healthcare standards.
Method
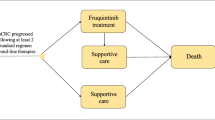
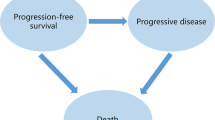
This study utilized data from the FRESCO-2 trial, incorporating a partitioned-survival model to simulate three health states: Progression-Free Survival, Progressive Disease, and death. Costs and utility values were derived from published literature and the FRESCO-2 trial. Sensitivity analyses were conducted to assess the robustness of the base-case result and to understand the impact of various parameters on the ICER.
Results
The base-case analysis revealed a total cost of $11,089.05 for the fruquintinib group and $5,374.48 for the placebo group. The overall QALYs were higher in the fruquintinib group (0.61 QALYs) compared to the placebo group (0.43 QALYs). The ICER was calculated to be $31,747.67 per QALY. Sensitivity analyses identified the utility of progression-free survival, the cost of fruquintinib, and the costs of best supportive care as significant determinants of ICER.
Conclusion
Fruquintinib emerges as a promising therapeutic option for refractory mCRC. However, its cost-effectiveness depends on selected willingness-to-pay (WTP) threshold. While the drug’s ICER surpasses the WTP based on China's 2022 GDP per capita, it remains below the threshold set at three times the national GDP.





Similar content being viewed by others
References
Sung H, Ferlay J, Siegel RL, et al. Global cancer statistics 2020: GLOBOCAN estimates of incidence and mortality worldwide for 36 cancers in 185 countries. CA: Cancer J Clin. 2021;71(3):209–49.
Cancer stat facts: Colorectal cancer. https://seer.cancer.gov/statfacts/html/colorect.html. Accessed 28 Aug 2023.
Xia C, Dong X, Li H, et al. Cancer statistics in China and United States, 2022: profiles, trends, and determinants. Chin Med J. 2022;135(5):584–90.
Jawed I, Wilkerson J, Prasad V, et al. Colorectal cancer survival gains and novel treatment regimens: A systematic review and analysis. JAMA Oncol. 2015;1(6):787–95.
Shen C, Tannenbaum D, Horn R, et al. Overall survival in phase 3 clinical trials and the surveillance, epidemiology, and end results database in patients with metastatic colorectal cancer, 1986–2016: a systematic review. JAMA Netw Open. 2022;5(5): e2213588.
Tang YL, Li DD, Duan JY, et al. Resistance to targeted therapy in metastatic colorectal cancer: current status and new developments. World J Gastroenterol. 2023;29(6):926–48.
Li J, Qin S, Xu RH, et al. Effect of fruquintinib vs placebo on overall survival in patients with previously treated metastatic colorectal cancer: the FRESCO randomized clinical trial. JAMA. 2018;319(24):2486–96.
Dasari A, Lonardi S, Garcia-Carbonero R, et al. Fruquintinib versus placebo in patients with refractory metastatic colorectal cancer (FRESCO-2): an international, multicentre, randomised, double-blind, phase 3 study. Lancet (Lond, Engl). 2023;402(10395):41–53.
Zhang PF, Xie D, Li Q. Cost-effectiveness analysis of fruquintinib as third-line treatment for patients with metastatic colorectal cancer. Tumori. 2020;106(5):400–5.
Peng Z, Hou X, Huang Y, et al. Cost-effectiveness analysis of fruquintinib for metastatic colorectal cancer third-line treatment in China. BMC Cancer. 2020;20(1):990.
Zhang Y, Wushouer H, Han S, et al. The impacts of government reimbursement negotiation on targeted anticancer medication price, volume and spending in China. BMJ Glob Health. 2021;6(7):e006196.
Murray CJ, Evans DB, Acharya A, et al. Development of WHO guidelines on generalized cost-effectiveness analysis. Health Econ. 2000;9(3):235–51.
Guyot P, Ades AE, Ouwens MJ, et al. Enhanced secondary analysis of survival data: reconstructing the data from published Kaplan–Meier survival curves. BMC Med Res Methodol. 2012;12:9.
Chongqing T, Sini L, Xiaohui Z, et al. Cost-effectiveness of first-line versus second-line pembrolizumab or chemotherapy in patients with microsatellite-instability-high/mismatch repair-deficient advanced colorectal cancer. Front Pharmacol. 2021;12: 802942.
Guan X, Li H, Xiong X, et al. Cost-effectiveness analysis of fruquintinib versus regorafenib as the third-line therapy for metastatic colorectal cancer in China. J Med Econ. 2021;24(1):339–44.
Ma Y, Zhou J, Ye Y, et al. The cost-effectiveness analysis of serplulimab versus regorafenib for treating previously treated unresectable or metastatic microsatellite instability-high or deficient mismatch repair colorectal cancer in China. Front Oncol. 2023;13:1113346.
Zhou T, Cao Y, Wang X, et al. Economic evaluation of sintilimab plus bevacizumab versus sorafenib as a first-line treatment for unresectable hepatocellular carcinoma. Adv Ther. 2022;39(5):2165–77.
Qin S, Kruger E, Tan SC, et al. Cost-effectiveness analysis of FOLFOX4 and sorafenib for the treatment of advanced hepatocellular carcinoma in China. Cost Eff Resource Alloc C/E. 2018;16:29.
Lang Y, Dong D, Wu B. Pembrolizumab vs the EXTREME regimen in recurrent or metastatic head and neck squamous cell carcinoma: a cost-effectiveness analysis. Clin Drug Investig. 2020;40(12):1137–46.
Li J, Qin S, Xu R, et al. Regorafenib plus best supportive care versus placebo plus best supportive care in Asian patients with previously treated metastatic colorectal cancer (CONCUR): a randomised, double-blind, placebo-controlled, phase 3 trial. Lancet Oncol. 2015;16(6):619–29.
Nafees B, Lloyd AJ, Dewilde S, et al. Health state utilities in non-small cell lung cancer: an international study. Asia Pac J Clin Oncol. 2017;13(5):e195–203.
Lloyd A, Nafees B, Narewska J, et al. Health state utilities for metastatic breast cancer. Br J Cancer. 2006;95(6):683–90.
Leech AA, Kim DD, Cohen JT, et al. Use and misuse of cost-effectiveness analysis thresholds in low- and middle-income countries: trends in cost-per-DALY studies. Value Health J Int Soc Pharmacoecon Outcomes Res. 2018;21(7):759–61.
Pichon-Riviere A, Drummond M, Palacios A, et al. Determining the efficiency path to universal health coverage: cost-effectiveness thresholds for 174 countries based on growth in life expectancy and health expenditures. Lancet Glob Health. 2023;11(6):e833–42.
Ochalek J, Wang H, Gu Y, et al. Informing a cost-effectiveness threshold for health technology assessment in China: a marginal productivity approach. Pharmacoeconomics. 2020;38(12):1319–31.
NICE. PMG19 Addendum A—final amendments to the NICE technology appraisal processes and methods guides to support the proposed new Cancer Drugs Fund arrangements. NICE London. 2016.
Prager GW, Taieb J, Fakih M, et al. Trifluridine–tipiracil and bevacizumab in refractory metastatic colorectal cancer. N Engl J Med. 2023;388(18):1657–67.
Voutsadakis IA. A systematic review and meta-analysis of trifluridine/tipiracil plus bevacizumab for the treatment of metastatic colorectal cancer: evidence from real-world series. Curr Oncol. 2023;30(6):5227–39.
Acknowledgements
This research was supported by National Key Clinical Specialties Construction Program.
Funding
No specific funding was received.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflicts of interest
The authors have no conflicts of interest to declare.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary Information
Below is the link to the electronic supplementary material.
Rights and permissions
Springer Nature or its licensor (e.g. a society or other partner) holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Huang, Z., Zhou, L., Zheng, H. et al. Cost-effectiveness analysis of fruquintinib in Chinese patients with refractory metastatic colorectal cancer. Int J Clin Pharm (2024). https://doi.org/10.1007/s11096-024-01721-1
Received:
Accepted:
Published:
DOI: https://doi.org/10.1007/s11096-024-01721-1