Abstract
Background
Although acetaminophen is recommended for the treatment of mild-to-moderate cancer pain, acetaminophen-induced hepatic disorders pose an important clinical challenge. Concomitant prescription of immune checkpoint inhibitors (ICIs) may further increase the risk of hepatic disorders in patients taking acetaminophen; however, there are few clinical studies that confirm this.
Aim
To evaluate the risk of hepatic disorders in patients taking concomitant acetaminophen and ICIs using a disproportionality analysis from the Japanese Adverse Drug Event Report database.
Method
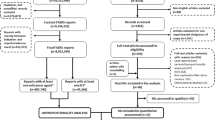
Acetaminophen users aged ≥ 20 years were included; factors that can affect the risk of acetaminophen-induced hepatic disorders were collated. Similar data on the widely used analgesic, loxoprofen, were used for comparison.
Results
Among 233,594 patients surveyed, 10,403 were prescribed acetaminophen, and among them, 1,245 patients developed hepatic disorders. The disproportionality of hepatic disorders was observed in acetaminophen users regardless of concomitant ICI use (without ICI: reporting odds ratio [ROR], 1.18; 95% confidence intervals [CI], 1.10–1.26; with ICI: ROR 1.87, 95%CI 1.59–2.20); it was even higher in concomitant acetaminophen and ICI users (ROR 1.94, 95%CI 1.65–2.29). However, increased disproportionality of hepatic disorders was not observed in patients taking concomitant loxoprofen and ICI. Multivariable logistic regression showed that the risk of hepatic disorders in acetaminophen users was associated with concomitant use of ICI (ROR, 1.91; 95% CI, 1.49–2.45); (P < 0.01).
Conclusion
Our findings suggest that the risk of hepatic disorders is greater with concomitant acetaminophen and ICI treatment than with acetaminophen alone.

Similar content being viewed by others
References
Aminoshariae A, Khan A. Acetaminophen: old drug, new issues. J Endod. 2015;41:588–93. https://doi.org/10.1016/j.joen.2015.01.024.
WHO. WHO guidelines for the pharmacological and radiotherapeutic management of cancer pain in adults and adolescents. https://www.who.int/publications/i/item/9789241550390. Accessed 01 Jan 2022.
Ishitsuka Y, Kondo Y, Kadowaki D. Toxicological property of acetaminophen: the dark side of a safe antipyretic/analgesic drug? Biol Pharm Bull. 2020;43:195–206. https://doi.org/10.1248/bpb.b19-00722.
Ramachandran A, Jaeschke H. Acetaminophen hepatotoxicity. Semin Liver Dis. 2019;39:221–34. https://doi.org/10.1055/s-0039-1679919.
Yan M, Huo Y, Yin S, et al. Mechanisms of acetaminophen-induced liver injury and its implications for therapeutic interventions. Redox Biol. 2018;17:274–83. https://doi.org/10.1016/j.redox.2018.04.019.
Jaeschke H, Ramachandran A. Mechanisms and pathophysiological significance of sterile inflammation during acetaminophen hepatotoxicity. Food Chem Toxicol. 2020;138:111240. https://doi.org/10.1016/j.fct.2020.111240.
Noda T, Kato R, Hattori T, et al. Role of caspase-8 and/or – 9 as biomarkers that can distinguish the potential to cause toxic- and immune related-adverse event, for the progress of acetaminophen-induced liver injury. Life Sci. 2022;294:120351. https://doi.org/10.1016/j.lfs.2022.120351.
Brahmer JR, Lacchetti C, Schneider BJ, et al. Management of immune-related adverse events in patients treated with immune checkpoint inhibitor therapy: american society of clinical oncology clinical practice guideline. J Clin Oncol. 2018;36:1714–68. https://doi.org/10.1200/JCO.2017.77.6385.
Thompson JA. New NCCN guidelines: recognition and management of immunotherapy-related toxicity. J Natl Compr Canc Netw. 2018;16:594–96. https://doi.org/10.6004/jnccn.2018.0047.
Pardoll DM. The blockade of immune checkpoints in cancer immunotherapy. Nat Rev Cancer. 2012;12:252–64. https://doi.org/10.1038/nrc3239.
Cho T, Kok LY, Uetrecht J. Testing possible risk factors for idiosyncratic drug-induced liver injury using an amodiaquine mouse model and co-treatment with 1-methyl-d-tryptophan or acetaminophen. ACS Omega. 2021;6:4656–62. https://doi.org/10.1021/acsomega.0c05352.
Pharmaceuticals and Medical Devices Agency. http://www.pmda.go.jp/. Accessed 07 Nov 2021.
Corcoran GB, Wong BK. Obesity as a risk factor in drug-induced organ injury: increased liver and kidney damage by acetaminophen in the obese overfed rat. J Pharmacol Exp Ther. 1987;241:921–27.
Michaut A, Moreau C, Robin MA, et al. Acetaminophen-induced liver injury in obesity and nonalcoholic fatty liver disease. Liver Int. 2014;34:e171-79. https://doi.org/10.1111/liv.12514.
Nguyen GC, Sam J, Thuluvath PJ. Hepatitis C is a predictor of acute liver injury among hospitalizations for acetaminophen overdose in the United States: a nationwide analysis. Hepatology. 2008;48:1336–41. https://doi.org/10.1002/hep.22536.
Tsumura H, Tamura I, Tanaka H, et al. Prescription of nonsteroidal anti-inflammatory drugs and co-prescribed drugs for mucosal protection: analysis of the present status based on questionnaires obtained from orthopedists in Japan. Intern Med. 2007;46:927–31. https://doi.org/10.2169/internalmedicine.46.0003.
Pharmaceutical and Medical Device Regulatory Science Society of Japan. MedDRA Japanese maintenance organization. https://www.jmo.pmrj.jp/. Accessed 02 May 2022.
FDA. Drug development and drug interactions. Table of substrates, inhibitors and inducers; 2019. https://www.fda.gov/drugs/drug-interactions-labeling/drug-development-and-drug-interactions-table-substrates-inhibitors-and-inducers#table3-3. Accessed 02 May 2022.
Guggenheimer J, Moore PA. The therapeutic applications of and risks associated with acetaminophen use: a review and update. J Am Dent Assoc. 2011;142:38–44. https://doi.org/10.14219/jada.archive.2011.0026.
Japan Society for the study of obesity (JASSO). Guidelines for the management of obesity disease. Tokyo: Life Science Publishing Co, Ltd; 2016.
AYUMI Pharmaceutical Corporation. CALONAL Package Inesrt Jpn. 2021.
WHO Collaborating Centre for Drug Statistics Methodology. ATC/DDD index 2022. https://www.whocc.no/atc_ddd_index/. Accessed 12 May 2022.
Cho YA, Han JM, Kang SY, et al. Analysis of risk factors for hepatotoxicity induced by immune checkpoint inhibitors. J Immunother. 2021;44:16–21. https://doi.org/10.1097/CJI.0000000000000347.
Greig SL, Garnock-Jones KP. Loxoprofen: a review in pain and inflammation. Clin Drug Investig. 2016;36:771–81. https://doi.org/10.1007/s40261-016-0440-9.
Aiso M, Takikawa H, Tsuji K, et al. Analysis of 307 cases with drug-induced liver injury between 2010 and 2018 in Japan. Hepatol Res. 2019;49:105–10. https://doi.org/10.1111/hepr.13288.
Acknowledgements
Editage (https://www.editage.jp/) provided assistance in the preparation of this manuscript.
Funding
This research did not receive any specific grant from funding agencies in the public, commercial, or not-for-profit sectors.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflicts of interest
The authors have no conflicts of interest to declare.
Additional information
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary Information
Below is the link to the electronic supplementary material.
Rights and permissions
Springer Nature or its licensor (e.g. a society or other partner) holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Yamada, T., Kato, R., Ijiri, Y. et al. Disproportionality analysis of acetaminophen-induced hepatic disorders with and without immune checkpoint inhibitors. Int J Clin Pharm 45, 442–450 (2023). https://doi.org/10.1007/s11096-022-01527-z
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11096-022-01527-z