Abstract
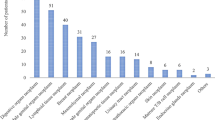
Background Several studies have examined the drug–drug interaction patterns in different patient populations and treatment settings; however, there is a need, particularly in the field of oncology and radiotherapy, for evaluating methods targeted towards preventing potential drug–drug interactions. One of the measures proposed is identifying potential interactions using computer programs and their evaluation by pharmacologists or clinical pharmacists, thereby providing clinically relevant information to the treating physician regarding the required prescription changes. Objective To determine the prevalence of potential drug–drug interactions in patients receiving chemoradiotherapy and assess the usefulness of expert team recommendations in minimizing interactions. Setting Patients admitted to the radiotherapy and oncology ward of a tertiary care teaching hospital in Karnataka, India. Method We conducted a prospective, cross-sectional study of prescriptions written for patients receiving chemoradiotherapy. Prescriptions containing two or more drugs, at least one of the drugs being an anticancer drug, were analyzed. They were screened for potential drug–drug interactions using the Lexicomp® drug interaction software. The interactions were classified as X, drug combination to be avoided; D, modification of therapy to be considered; and C, therapy to be monitored, as per the Lexicomp criteria. Main outcome measure The number of drug–drug interactions detected that were accepted by the treating radio-oncologist as requiring prescription change before and after the prescription review by an expert team. Results Two hundred twenty-three prescriptions were screened for the presence of drug–drug interactions; 106 prescriptions (47.53%) containing 620 drugs and 211 drug–drug interactions were identified. Of the 211 interactions identified, 6.64% (14/211), 18.48% (39/211), and 74.88% (158/211) drug–drug interactions belonged to category X, D, and C, respectively. Twenty-seven (50.94%) of the 53 category X and D interactions identified were accepted the oncologist as requiring a change in the prescription; an additional 13 (24.53%) interactions were identified as significant by the expert team, and 11 (84.62%) of these were accepted by the oncologist. Conclusion A system of alerting the treating physician to a potential drug–drug interaction leads to avoidance of prescription of the interacting drug combination, and the assistance by an expert team adds significantly to avoidance of clinically relevant drug interactions.


Similar content being viewed by others
References
Magro L, Moretti U, Leone R. Epidemiology and characteristics of adverse drug reactions caused by drug–drug interactions. Expert Opin Drug Saf. 2012;11:83–94.
Greenblatt DJ. Mechanisms and consequences of drug–drug interactions. Clin Pharmacol Drug Dev. 2017;6:118–24.
Percha B, Altman RB. Informatics confronts drug–drug interactions. Trends Pharmacol Sci. 2013;34:178–84.
Strandell J, Bate A, Lindquist M, Edwards IR, Swedish, Finnish IXDID (SFINX G). Drug–drug interactions—a preventable patient safety issue? Br J Clin Pharmacol. 2008;65:144–6.
Guthrie B, Makubate B, Hernandez-Santiago V, Dreischulte T. The rising tide of polypharmacy and drug–drug interactions: population database analysis 1995–2010. BMC Med. 2015;13:74.
Kannan G, Anitha R, Rani VN, Thennarasu P, Alosh J, Vasantha J, et al. A study of drug–drug interactions in cancer patients of a south Indian tertiary care teaching hospital. J Postgrad Med. 2011;57:206–10.
van Leeuwen RWF, Jansman FGA, van den Bemt PMLA, de Man F, Piran F, Vincenten I, et al. Drug–drug interactions in patients treated for cancer: a prospective study on clinical interventions. Ann Oncol Off J Eur Soc Med Oncol. 2015;26:992–7.
van Leeuwen RWF, Brundel DHS, Neef C, van Gelder T, Mathijssen RHJ, Burger DM, et al. Prevalence of potential drug–drug interactions in cancer patients treated with oral anticancer drugs. Br J Cancer. 2013;108:1071–8.
O’Neill CB, Baxi SS, Atoria CL, O’Neill JP, Henman MC, Sherman EJ, et al. Treatment-related toxicities in older adults with head and neck cancer: a population-based analysis. Cancer. 2015;121:2083–9.
van Leeuwen RWF, Swart EL, Boven E, Boom FA, Schuitenmaker MG, Hugtenburg JG. Potential drug interactions in cancer therapy: a prevalence study using an advanced screening method. Ann Oncol Off J Eur Soc Med Oncol. 2011;22:2334–411.
Soldatos TG, Jackson DB. Adverse event circumstances and the case of drug interactions. Healthcare (Basel). 2019;7:45.
Roblek T, Vaupotic T, Mrhar A, Lainscak M. Drug–drug interaction software in clinical practice: a systematic review. Eur J Clin Pharmacol. 2015;71:131–42.
Meslin SMM, Zheng WY, Day RO, Tay EMY, Baysari MT. Evaluation of clinical relevance of drug–drug interaction alerts prior to implementation. Appl Clin Inform. 2018;9:849–55.
Nightingale G, Pizzi LT, Barlow A, Barlow B, Jacisin T, McGuire M, et al. The prevalence of major drug–drug interactions in older adults with cancer and the role of clinical decision support software. J Geriatr Oncol. 2018;9:526–33.
Girre V, Arkoub H, Puts MTE, Vantelon C, Blanchard F, Droz JP, et al. Potential drug interactions in elderly cancer patients. Crit Rev Oncol Hematol. 2011;78:220–6.
Lopez-Martin C, Garrido Siles M, Alcaide-Garcia J, Faus FV. Role of clinical pharmacists to prevent drug interactions in cancer outpatients: a single-centre experience. Int J Clin Pharm. 2014;36:1251–9.
Bhowmick S, Shenoy A. Evolving role of clinical pharmacologists in Indian accredited hospitals. J Pharmacol Pharmacother. 2018;9:121.
Deshpande PR, Vantipalli R, Chaitanya Lakshmi CH, Rao EJ, Regmi B, Ahad A, et al. Clinical pharmacists: the major support to Indian healthcare system in near future. J Pharm Bioallied Sci. 2015;7:161–74.
Lexicomp® drug interact [internet]. Wolters Kluwer-[cited 2019 Nov 8]. Available from: https://www.uptodate.com/drug-interactions/?source=responsive_home#di-druglist.
Mouzon A, Kerger J, D’Hondt L, Spinewine A. Potential interactions with anticancer agents: a cross-sectional study. Chemotherapy. 2013;59:85–92.
Riechelmann RP, Moreira F, Smaletz O, Saad ED. Potential for drug interactions in hospitalized cancer patients. Cancer Chemother Pharmacol. 2005;56:286–90.
Balk TE, van der Sijs IH, van Gelder T, Janssen JJB, van der Sluis IM, van Leeuwen RWF, et al. Drug–drug interactions in pediatric oncology patients. Pediatr Blood Cancer. 2017;64:e26410.
Vayalil R, Shetty KJ, Mateti U. Assessment of potential drug–drug interactions in an oncology unit of a tertiary care teaching hospital. Indian J Med Paediatr Oncol. 2018;39:436.
Chen L, Cheung WY. Potential drug interactions in patients with a history of cancer. Curr Oncol. 2014;21:e212–e220220.
Marcath LA, Coe TD, Hoylman EK, Redman BG, Hertz DL. Prevalence of drug–drug interactions in oncology patients enrolled on National Clinical Trials Network oncology clinical trials. BMC Cancer. 2018;18:1155.
Ramos-Esquivel A, Víquez-Jaikel Á, Fernández C. Potential drug–drug and herb–drug interactions in patients with cancer: a prospective study of medication surveillance. J Oncol Pract. 2017;13:e613–e622622.
Taegtmeyer AB, Kullak-Ublick GA, Widmer N, Falk V, Jetter A. Clinical usefulness of electronic drug–drug interaction checking in the care of cardiovascular surgery inpatients. Cardiology. 2012;123:219–22.
Das S, Behera SK, Xavier AS, Dharanipragada S, Selvarajan S. Are drug–drug interactions a real clinical concern? Perspect Clin Res. 2019;10:62–6.
Fung KW, Kapusnik-Uner J, Cunningham J, Higby-Baker S, Bodenreider O. Comparison of three commercial knowledge bases for detection of drug–drug interactions in clinical decision support. J Am Med Inform Assoc. 2017;24:806–12.
Ribed A, Romero-Jiménez RM, Escudero-Vilaplana V, Iglesias-Peinado I, Herranz-Alonso A, Codina C, et al. Pharmaceutical care program for onco-hematologic outpatients: safety, efficiency and patient satisfaction. Int J Clin Pharm. 2016;38:280–8.
Maleki S, Alexander M, Fua T, Liu C, Rischin D, Lingaratnam S. A systematic review of the impact of outpatient clinical pharmacy services on medication-related outcomes in patients receiving anticancer therapies. J Oncol Pharm Pract. 2019;25:130–9.
Mekonnen AB, McLachlan AJ, Brien JE. Effectiveness of pharmacist-led medication reconciliation programmes on clinical outcomes at hospital transitions: a systematic review and meta-analysis. BMJ Open. 2016;6:e010003.
Ussai S, Petelin R, Giordano A, Malinconico M, Cirillo D, Pentimalli F. A pilot study on the impact of known drug–drug interactions in cancer patients. J Exp Clin Cancer Res. 2015;34:89.
Bulsink A, Imholz ALT, Brouwers JRBJ, Jansman FGA. Characteristics of potential drug-related problems among oncology patients. Int J Clin Pharm. 2013;35:401–7.
Buffery PJ, Strother RM. Domperidone safety: a mini-review of the science of QT prolongation and clinical implications of recent global regulatory recommendations. N Z Med J. 2015;128:66–74.
Biewenga J, Keung C, Solanki B, Natarajan J, Leitz G, Deleu S, et al. Absence of QTc prolongation with domperidone: a randomized, double-blind, placebo-and positive-controlled thorough QT/QTc study in healthy volunteers. Clin Pharmacol Drug Dev. 2015;4:41–8.
Bor S, Demir M, Ozdemir O, Yuksel K. A meta-analysis on the cardiac safety profile of domperidone compared to metoclopramide. United Eur Gastroenterol J. 2018;6:1331–466.
Hesketh PJ, Kris MG, Basch E, Bohlke K, Barbour SY, Clark-Snow RA, et al. Antiemetics: American society of clinical oncology clinical practice guideline update. J Clin Oncol. 2017;35:3240–61.
Product monograph. Iressa@ gefitinib tablets epidermal growth factor receptor (EGFR) tyrosine kinase inhibitor [internet]. 2017 [cited 2019 Nov 8]. Available from: https://www.astrazeneca.ca/content/dam/az-ca/downloads/productinformation/iressa-product-monograph-en.pdf.
Acknowledgements
None.
Funding
The study did not receive any funding from governmental or non-governmental sources.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflicts of interest
The authors declare that they have no competing interests.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
About this article
Cite this article
Daggupati, S.J.V., Saxena, P.P., Kamath, A. et al. Drug–drug interactions in patients undergoing chemoradiotherapy and the impact of an expert team intervention. Int J Clin Pharm 42, 132–140 (2020). https://doi.org/10.1007/s11096-019-00949-6
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11096-019-00949-6