Abstract
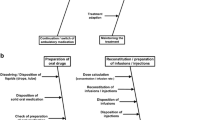
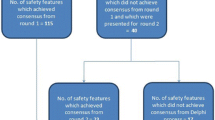
Background Medicines should be compounded by using an aseptic technique to assure patient safety. The parenteral administration of microbiologically contaminated doses can result in bacteriaemia, other morbidity and even death. Objective The purpose was to develop and content validate an assessment tool for medicine compounding on hospital wards suitable for self-assessment and external audit to ensure the safety of medicine compounding on wards. Setting Finland as setting. Method The first draft of the tool was based on ISMP “Guidelines for safe preparation of sterile compounds” and a systematic literature search. The tool was validated by using a two-rounded Delphi-method with a panel of 19 experts. Suitability and feasibility of each item was evaluated. Main outcome measure An agreement of ≥70% on each item was required. Results The final tool comprises of 64 items under the following topics: (1) general principles of good compounding practices (23 items), (2) recording and confirming medicine orders on the wards (5 items), (3) storage of medicines on the wards (7), (4) aseptic compounding of intravenous medicines (25 items) and (5) quality assurance (4 items). Most items related to General principles of good compounding practices and Compounding of IV medicines (36 and 38% of the items, respectively). Conclusion It was possible to develop and content validate, by the Delphi method, an assessment tool for safe aseptic compounding on hospital wards. A two-round Delphi process yielded consensus on 64 items for this purpose.

Similar content being viewed by others
References
Noskin GA, Rubin RJ, Schentag JJ, Kluytmans J, Hedblom EC, Smulders M, et al. The Burden of Staphylococcus aureus infections in hospitals in the United States: an analysis of the 2000 and 2001 nationwide inpatient sample database. Arch Intern Med. 2005;165:1756–61.
National Nosocomical Infections Surveillance System. National nosocomical infections surveillance (NNIS) system report, data summary from January 1992 through June 2004, issued October 2004. Am J Infect Control. 2004;32:470–85.
Institute for Safe Medication Practices (ISMP): guidelines for safe preparation of sterile compounds, 2013. http://www.ismp.org/tools/guidelines/IVSummit/IVCGuidelines.pdf. Accessed 29 Feb 2016.
Olivier LC, Kendoff D, Wolfhard U, Nast-Kolb D, Nazif Yazici M, Esche H. Modified syringe design prevents plunger-related contamination-results of contamination and flow rate tests. J Hosp Infect. 2003;53:140–3.
Sharp J. Quality in the manufacture of medicines and other healthcare products. London: Pharmaceutical Press; 2000. p. 331–60.
Farwell J. Aseptic dispensing for NHS patients (Farwell report). London: Department of Health; 1995.
The Finnish Medicines Agency (Fimea). Määräys 6/2011, Apteekkien lääkevalmistus (Requirement 6/2011: preparation of medicines in pharmacies). http://www.fimea.fi/documents/160140/764653/20675_FINAL_Apteekkien_laakevalmistus_maarays_SUOMI_2011-12-16.pdf. Accessed 29 Feb 2016.
The Finnish Medicines Agency (Fimea). Määräys 6/2012, Sairaala-apteekin ja lääkekeskuksen toiminta (Requirement 6/2012: Function of hospital pharmacies and dispensaries). http://www.fimea.fi/documents/160140/764653/22690_Maarays_6_2012.pdf. Accessed 29 Feb 2016.
Garqiulo DA, Sheridan J, Webster CS, Swift S, Torrie J, Weller J, et al. Anaesthetic drug administration as a potential contributor to healthcare-associated infections: a prospective simulation-based evaluation of aseptic techniques in the administration of anaesthetic drugs. BMJ Qual Saf. 2012;21:826–34.
Hsu C-C, Sandford BA. The Delphi technique: making sense of consensus. Pract Assess Res Eval. 2007;12(10):1–7.
Campbell SM, Cantrill JA. Consensus methods in prescribing research. J Clin Pharm Ther. 2001;26:5–14.
Definition of Webropol. http://www.webropol.fi/. Accessed 29 Feb 2016.
Keeney S, Hasson F, Mckenna H. The Delphi technique in nursing and health research. Hoboken: Wiley; 2010.
European commission. EU guidelines to good manufacturing practice medicinal products for human and veterinary use. 2008; 4 Annex 1: manufacture of sterile medicinal products (corrected version) http://ec.europa.eu/health/files/eudralex/vol-4/2008_11_25_gmp-an1_en.pdf. Accessed 29 Feb 2016.
Dougherty L, Lister S. The Royal Marsden Hospital Manual of Clinical Nursing procedures. 8th ed. Chichester: Wiley; 2012.
Koskinen T, Ojala R, Puirava A, Puirava P, Sälimäki J. Lääketietoa ammattilaisille (Drug information for professionals). 1st ed. Helsinki: Sanoma Pro Oy; 2012.
Nurminen ML. Lääkehoito (Medication). 9th ed. Helsinki: Sanoma Pro Oy; 2008.
Taam-Ukkonen M, Saano S. Turvallisen lääkehoidon perusteet (Principles of Safe Medication). 8th ed. Helsinki: Sanoma Pro Oy; 2016.
Saano S, Naaranlahti T, Helin-Tanninen M, Järviluoma E. Sairaalafarmasia (Hospital Pharmacy). 1st ed. Kuopio: Farmasian opiskelijayhdistys Fortis ry; 2005.
Veräjänkorva O, Huupponen R, Huupponen U, Kaukkila H-S, Torniainen K. Lääkehoito hoitotyössä (Pharmacotherapy in Nursing). 1st ed. Helsinki: WSOY; 2006.
Saano S, Taam-Ukkonen M. Lääkehoidon käsikirja (Handbook of Pharmacotherapy). 1st ed. Helsinki: Sanoma Pro Oy; 2013.
Karhumäki E, Jonsson A, Saros M. Mikrobit hoitotyön haasteena (Microbes challenge for nursing). 2nd ed. Helsinki: Edita Prima Oy; 2009.
Austin P, Elia M. Improved aseptic technique can reduce variable contamination rates of ward-prepared parenteral doses. J Hosp Infect. 2013;83:160–3.
De Smet B, Veng C, Kruy L, Kham C, van Griensven C, Peeters C, et al. Outbreak of Burkholderia cepacia bloodstream infections traced to the use of Ringer lactate solution as multiple-dose vial for catheter flushing, Phnom Penh, Cabodia. Clin Microbiol Infect. 2013;19:832–7.
Cousins DH, Sabatier B, Beque D, Schmitt C, Hoppe-Tichy T. Medication errors in intravenous drug preparation and administration: a multicentre audit in the UK, Germany and France. Qual Saf Health Care. 2005;14:190–5.
Frean JA, Arntzen L, Rosekilly I, Isaäcson M. Investigation of contaminated parenteral nutrition fluids associated with an outbreak of serratia odorifera septicaemia. J Hosp Infect. 1994;27:263–73.
McCrea MK. No excuse for unsafe injection practices. AORN J. 2013;97:132–5.
Campbell SM, Cantrill JA. Consensus methods in prescribing research. J Clin Pharm Ther. 2001;26:5–14.
Fick DM, Cooper JW, Wade WE, Waller JL, Maclean JR, Beers MH. Updating the Beers criteria for potentially inappropriate medication use in older adults: results of US consensus panel of experts. Arch Intern Med. 2003;163:2716–24.
Laroche ML, Charmes JP, Merle L. Potentially inappropriate medications in the elderly: a French consensus panel list. Eur J Clin Pharmacol. 2007;63:725–31.
Dimitrow MS, Mykkänen SI, Leikola SN, Kivelä SL, Lyles A, Airaksinen MS. Content validation of a tool for assessing risks for drug-related problems to be used by practical nurses caring for home-dwelling clients aged ≥65 years: a Delphi survey. Eur J Clin Pharmacol. 2014;70:991–1002.
Teinilä T, Halmepuro-Jaatinen S, Yritys K, Manni K, Airaksinen M. Adapting the US Institute for Safe Medication Practices’ Medication Safety Self-Assessment tool for community pharmacies in Finland. Int J Pharm Pract. 2012;20:15–24.
Celikkayalar E, Myllyntausta M, Grissinger M, Airaksinen M. Adapting and remodelling the US Institute for Safe Medication Practices’ Medication Safety Self-Assessment tool for hospitals to be used to support natuonal medication safety initiatives in Finland. Int J Pharm Pract. 2016;24(4):262–70.
Acknowledgements
Corresponding author was supported by the state research funding granted by Satakunta Hospital District when completing this manuscript.
Funding
None.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflicts of interest
None.
Electronic supplementary material
Below is the link to the electronic supplementary material.
Rights and permissions
About this article
Cite this article
Suvikas-Peltonen, E., Granfors, E., Celikkayalar, E. et al. Development and content validation of an assessment tool for medicine compounding on hospital wards. Int J Clin Pharm 38, 1457–1463 (2016). https://doi.org/10.1007/s11096-016-0389-z
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11096-016-0389-z