Abstract
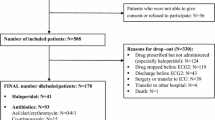
Introduction Drug-induced QT-prolongation is an established risk factor for Torsade de pointes and sudden cardiac death. The list of QT-prolonging drugs is extensive and includes many drugs commonly used in psychiatry. Aim In this study we performed a cross-sectional analysis of medication profiles to assess the prevalence of drug interactions potentially leading to QT-prolongation. Setting 6 psychiatric hospitals in Flanders, Belgium. Methods For each patient, the full medication list was screened for the presence of interactions, with special attention to those with an increased risk for QT-prolongation. Current practice on QT monitoring and prevention of drug-induced arrhythmia was assessed. Main outcome measure Number of drug interactions with risk of QT-prolongation. Results 592 patients (46 % female; mean age 55.7 ± 17.1 years) were included in the analysis. 113 QT-prolonging interactions were identified in 43 patients (7.3 %). QT-prolonging interactions occurred most frequently with antidepressants (n = 102) and antipsychotics (n = 100). The precautions and follow-up provided by the different institutions when combining QT-prolonging drugs were very diverse. Conclusion Drug combinations that are associated with QT-prolongation are frequently used in the chronic psychiatric setting. Persistent efforts should be undertaken to provide caregivers with clear guidelines on how to use these drugs in a responsible and safe way.

Similar content being viewed by others
References
Josephson M, Wellens HJ. Implantable defibrillators and sudden cardiac death. Circulation. 2004;109:2685–91.
Myerburg RJ, Kessler KM, Castellanos A. Sudden cardiac death: epidemiology, transient risk, and intervention assessment. Ann Intern Med. 1993;119:1187–97.
Myerburg RJ, Interian A Jr, Mitrani RM, Kessler KM, Castellanos A. Frequency of sudden cardiac death and profiles of risk. Am J Cardiol. 1997;80:10F–9F.
van Noord C, Eijgelsheim M, Stricker BH. Drug- and non-drug-associated QT interval prolongation. Br J Clin Pharmacol. 2010;70:16–23.
Drew BJ, Ackerman MJ, Funk M, Gibler WB, Kligfield P, Menon V, et al. Prevention of Torsade de pointes in hospital settings: a scientific statement from the American Heart Association and the American College of Cardiology Foundation. J Am Coll Cardiol. 2010;55:934–47.
Roden DM. Drug-induced prolongation of the QT interval. N Engl J Med. 2004;350:1013–22.
U.S. Department of Health and Human Services. Guidance for industry: E14 clinical evaluation of QT/QTc interval prolongation and proarrhythmic potential for non-antiarrhythmic drugs. 2005.
Davey P. How to correct the QT interval for the effects of heart rate in clinical studies. J Pharmacol Toxicol Methods. 2002;48:3–9.
Arizona Center for Education and Research on Therapeutics (AZCERT): QT drug lists by risk groups. 2013. http://www.crediblemeds.org (last accessed 19 Dec 2013) (online).
De Bruin ML, Pettersson M, Meyboom RH, Hoes AW, Leufkens HG. Anti-HERG activity and the risk of drug-induced arrhythmias and sudden death. Eur Heart J. 2005;26:590–7.
Straus SM, Sturkenboom MC, Bleumink GS, Dieleman JP, van der Lei J, de Graeff PA, et al. Non-cardiac QTc-prolonging drugs and the risk of sudden cardiac death. Eur Heart J. 2005;26:2007–12.
Armahizer MJ, Seybert AL, Smithburger PL, Kane-Gill SL. Drug-drug interactions contributing to QT prolongation in cardiac intensive care units. J Crit Care. 2013;28:243–9.
Jolly K, Gammage MD, Cheng KK, Bradburn P, Banting MV, Langman MJ. Sudden death in patients receiving drugs tending to prolong the QT interval. Br J Clin Pharmacol. 2009;68:743–51.
Ray WA, Chung CP, Murray KT, Hall K, Stein CM. Atypical antipsychotic drugs and the risk of sudden cardiac death. N Engl J Med. 2009;360:225–35.
Leonard CE, Freeman CP, Newcomb CW, Bilker WB, Kimmel SE, Strom BL, et al. Antipsychotics and the risks of sudden cardiac death and all-cause death: cohort studies in medicaid and dually-eligible medicaid–medicare beneficiaries of five states. J Clin Exp Cardiol. 2013;10:1–9.
Beach SR, Celano CM, Noseworthy PA, Januzzi JL, Huffman JC. QTc prolongation, Torsades de Pointes, and psychotropic medications. Psychosomatics. 2013;54:1–13.
Castro VM, Clements CC, Murphy SN, Gainer VS, Fava M, Weilburg JB, et al. QT interval and antidepressant use: a cross sectional study of electronic health records. BMJ. 2013;346:288.
Leonard CE, Bilker WB, Newcomb C, Kimmel SE, Hennessy S. Antidepressants and the risk of sudden cardiac death and ventricular arrhythmia. Pharmacoepidemiol Drug Saf. 2011;20:903-913.
Moller HJ, Seemuller F, Schennach-Wolff R, Stubner S, Ruther E, Grohmann R. History, background, concepts and current use of comedication and polypharmacy in psychiatry. Int J Neuropsychopharmacol. 2013;18:1–14.
Seemuller F, Riedel M, Obermeier M, Bauer M, Adli M, Kronmuller K, et al. Outcomes of 1014 naturalistically treated inpatients with major depressive episode. Eur Neuropsychopharmacol. 2010;20:346–55.
Stubner S, Grohmann R, von SI, Ruther E, Moller HJ, Muller-Oerlinghausen B, et al. Suicidality as rare adverse event of antidepressant medication: report from the AMSP multicenter drug safety surveillance project. J Clin Psychiatry. 2010;71:1293–307.
Patel MX, Bishara D, Jayakumar S, Zalewska K, Shiers D, Crawford MJ, et al. Quality of prescribing for schizophrenia: evidence from a national audit in England and Wales. Eur Neuropsychopharmacol. 2014;24:499–509.
Sala M, Vicentini A, Brambilla P, Montomoli C, Jogia JR, Caverzasi E, et al. QT interval prolongation related to psychoactive drug treatment: a comparison of monotherapy versus polytherapy. Ann Gen Psychiatry. 2005;4:1.
Barnes T, Davison S, Ferrier IN, Howard R, Kerwin R, King DJ, et al. Consensus statement on high-dose antipsychotic medication. London: Royal College of Psychiatrists. 2005.
Ames D, Camm J, Cook P, Falkai P, Gury C, Hurley R, et al. Minimizing the risks associated with QTc prolongation in people with schizophrenia. A consensus statement by the Cardiac Safety in Schizophrenia Group. Encephale. 2002;28:552–62.
Ozeki Y, Fujii K, Kurimoto N, Yamada N, Okawa M, Aoki T, et al. QTc prolongation and antipsychotic medications in a sample of 1017 patients with schizophrenia. Prog Neuropsychopharmacol Biol Psychiatry. 2010;34:401–5.
Reilly JG, Ayis SA, Ferrier IN, Jones SJ, Thomas SH. QTc-interval abnormalities and psychotropic drug therapy in psychiatric patients. Lancet. 2000;355:1048–52.
WHO Collaborating Centre for Drug Statistics Methodology. ATC/DDD index. 2013. http://www.whocc.no/atc_ddd_index (last accessed 15 Apr 2014) (online).
Curtis LH, Ostbye T, Sendersky V, Hutchison S, Allen LaPointe NM, Al-Khatib SM, et al. Prescription of QT-prolonging drugs in a cohort of about 5 million outpatients. Am J Med. 2003;114:135–41.
Haueis P, Greil W, Huber M, Grohmann R, Kullak-Ublick GA, Russmann S. Evaluation of drug interactions in a large sample of psychiatric inpatients: a data interface for mass analysis with clinical decision support software. Clin Pharmacol Ther. 2011;90:588–96.
Abarca J, Malone DC, Armstrong EP, Grizzle AJ, Hansten PD, Van Bergen RC, et al. Concordance of severity ratings provided in four drug interaction compendia. J Am Pharm Assoc. 2004;44:136–41.
Vitry AI. Comparative assessment of four drug interaction compendia. Br J Clin Pharmacol. 2007;63:709–14.
Wong CM, Ko Y, Chan A. Clinically significant drug-drug interactions between oral anticancer agents and nonanticancer agents: profiling and comparison of two drug compendia. Ann Pharmacother. 2008;42:1737–48.
Isaac T, Weissman JS, Davis RB, Massagli M, Cyrulik A, Sands DZ, et al. Overrides of medication alerts in ambulatory care. Arch Intern Med. 2009;169:305–11.
Weingart SN, Toth M, Sands DZ, Aronson MD, Davis RB, Phillips RS. Physicians’ decisions to override computerized drug alerts in primary care. Arch Intern Med. 2003;163:2625–31.
Kuperman GJ, Bobb A, Payne TH, Avery AJ, Gandhi TK, Burns G, et al. Medication-related clinical decision support in computerized provider order entry systems: a review. J Am Med Inform Assoc. 2007;14:29–40.
van der Sijs H, Kowlesar R, Klootwijk AP, Nelwan SP, Vulto AG, van GT. Clinically relevant QTc prolongation due to overridden drug–drug interaction alerts: a retrospective cohort study. Br J Clin Pharmacol. 2009;67:347–54.
Indermitte J, Beutler M, Bruppacher R, Meier CR, Hersberger KE. Management of drug-interaction alerts in community pharmacies. J Clin Pharm Ther. 2007;32:133–42.
Murphy JE, Forrey RA, Desiraju U. Community pharmacists’ responses to drug–drug interaction alerts. Am J Health Syst Pharm. 2004;61:1484–7.
Zivin K, Pfeiffer PN, Bohnert AS, Ganoczy D, Blow FC, Nallamothu BK, et al. Evaluation of the FDA warning against prescribing citalopram at doses exceeding 40 mg. Am J Psychiatry. 2013;170:642–50.
Narang P, El-Refai M, Parlapalli R, Danilov L, Manda S, Kaur G, et al. Antipsychotic drugs: sudden cardiac death among elderly patients. Psychiatry (Edgmont). 2010;7:25–9.
Drezner JA, Ackerman MJ, Cannon BC, Corrado D, Heidbuchel H, Prutkin JM, et al. Abnormal electrocardiographic findings in athletes: recognising changes suggestive of primary electrical disease. Br J Sports Med. 2013;47:153–67.
Nielsen J, Graff C, Kanters JK, Toft E, Taylor D, Meyer JM. Assessing QT interval prolongation and its associated risks with antipsychotics. CNS Drugs. 2011;25:473–90.
Acknowledgments
We would like to thank Delphine Lesage, Tinne Leyssens, Eline Sol, Nathalie Rahier, Pieter Ramaut, Annelies Van Huynegem and Hakima Abasbassi from the department of Pharmaceutical Sciences for their contribution to the data collection and analysis. We also want to thank the pharmacists of the 6 psychiatric institutions for their participation: Johan Reyntens, Sint-Jan hospital; Marc De Vos, Sint-Jan-Baptist hospital; Daniel Vrijders, Zoete Nood Gods hospital, Maria De Voght, Sint-Alexius hospital; Erik Pauwels, Sint-Lucia hospital, and Willy De Boever, Sint-Hieronymus hospital.
Funding
This work was supported by unconditional grants from Boston Scientific and Medtronic Belgium. Dr. Willems is supported as a clinical researcher by the Fund for Scientific Research Flanders. PhD-student Eline Vandael is supported by funding of the Belgian government agency for Innovation by Science and Technology (IWT).
Conflicts of interest
None.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Vandael, E., Marynissen, T., Reyntens, J. et al. Frequency of use of QT-interval prolonging drugs in psychiatry in Belgium. Int J Clin Pharm 36, 757–765 (2014). https://doi.org/10.1007/s11096-014-9953-6
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11096-014-9953-6