Abstract
Purpose
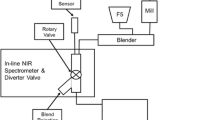
This study evaluates the use of the closed feed frame as a material sparing approach to develop near-infrared (NIR) spectroscopic calibration models for monitoring blend uniformity. The effect of shear induced by recirculation on NIR spectra was also studied.
Methods
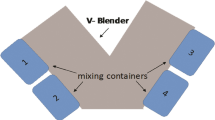
Calibration models were developed using NIR spectra obtained in the closed feed frame for two cases. For case 2, blends that flowed through the open feed frame were predicted with the model. The shear effect of the feed frame on the blends was assessed through the characterization of powder properties before and after recirculation.
Results
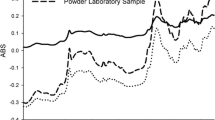
The physical characterization of the blends confirmed that the powder properties were not altered after recirculation within the closed feed frame. Both calibration models provided highly accurate predictions of the test sets with low bias (0.03% w/w and -0.06% w/w) and relative standard error of prediction (1.9% and 3.7%), respectively. The predictive performance of the calibration models was not affected by the shear effect.
Conclusion
Recirculation within the closed feed frame did not change the physical properties of the blends studied. The prediction of blends flowing through the open feed frame was possible with a calibration model developed in the closed feed frame. The closed feed frame could reduce the materials needed to develop calibration models by more than 90%.












Similar content being viewed by others
Data Availability
The data is available in the following data repository: https://github.com/Nmmeza07/Closed-Feed-Frame-data.
Abbreviations
- API:
-
Active pharmaceutical ingredient
- CM:
-
Continuous manufacturing
- MgSt:
-
Magnesium stearate
- MPE:
-
Minimal practical error
- NIR:
-
Near-infrared
- PLS:
-
Partial least squares
- RMSEP:
-
Root mean square error of prediction
- RSEP:
-
Relative standard error of prediction
- SNV:
-
Standard normal variate
References
Muzzio FJ, Oka S. How to design and implement powder-to-tablet continuous manufacturing systems. Elsevier; 2022. p. 1–413. https://doi.org/10.1016/C2016-0-03217-5.
Sierra-Vega NO, Romañach RJ, Méndez R. Feed frame: The last processing step before the tablet compaction in pharmaceutical manufacturing. Int J Pharm. 2019;572. https://doi.org/10.1016/j.ijpharm.2019.118728.
Velez NL, Drennen JK, Anderson CA. Challenges, opportunities and recent advances in near infrared spectroscopy applications for monitoring blend uniformity in the continuous manufacturing of solid oral dosage forms. Int J Pharm. 2022;615. https://doi.org/10.1016/j.ijpharm.2022.121462.
Rangel-Gil RS, Sierra-Vega NO, Romañach RJ, Méndez R. Assessment of blend uniformity in a stream sampler device using Raman spectroscopy. Int J Pharm. 2023;25(639). https://doi.org/10.1016/j.ijpharm.2023.122934
FDA. Guidance for Industry PAT — A Framework for Innovative Pharmaceutical. 2004. Available from: http://www.fda.gov/cvm/guidance/published.html. Accessed 17 Jul 2023.
Gupta A, Peck GE, Miller RW, Morris KR. Real-time near-infrared monitoring of content uniformity, moisture content, compact density, tensile strength, and young’s modulus of roller compacted powder blends. J Pharm Sci. 2005;94(7):1589–97. https://doi.org/10.1002/jps.20375.
Pérez-Ramos JD, Findlay WP, Peck G, Morris KR. Quantitative analysis of film coating in a pan coater based on in-line sensor measurements. AAPS PharmSciTech. 6(1):E127–36. https://doi.org/10.1208/pt060120
Morris KR, Stowell JG, Byrn SR, et al. Accelerated fluid bed drying using NIR monitoring and phenomenological modeling. Drug Development and Industrial Pharmacy. 2000;26:985–8. https://doi.org/10.1081/DDC-100101326.
Hetrick EM, Shi Z, Barnes LE, Garrett AW, Rupard RG, Kramer TT, et al. Development of near infrared spectroscopy-based process monitoring methodology for pharmaceutical continuous manufacturing using an offline calibration approach. Anal Chem. 2017;89(17):9175–83. https://doi.org/10.1021/acs.analchem.7b01907.
Sierra-Vega NO, Román-Ospino A, Scicolone J, Muzzio FJ, Romañach RJ, Méndez R. Assessment of blend uniformity in a continuous tablet manufacturing process. Int J Pharm. 2019;5(560):322–33. https://doi.org/10.1016/j.ijpharm.2019.01.073
Sierra-Vega NO, Sánchez-Paternina A, Maldonado N, Cárdenas V, Romañach RJ, Méndez R. In line monitoring of the powder flow behavior and drug content in a Fette 3090 feed frame at different operating conditions using Near Infrared spectroscopy. J Pharm Biomed Anal. 2018;154:384–96. https://doi.org/10.1016/j.jpba.2018.03.017.
Li Y, Anderson CA, Drennen JK, Airiau C, Igne B. Development of an in-line near-infrared method for blend content uniformity assessment in a tablet feed frame. Appl Spectrosc. 2019;73(9):1028–40. https://doi.org/10.1177/0003702819842189.
Ortega-Zúñiga C, la Rosa CP, De R-O, Serrano-Vargas A, Romañach RJ, Méndez R. Development of near infrared spectroscopic calibration models for in-line determination of low drug concentration, bulk density, and relative specific void volume within a feed frame. J Pharm Biomed Anal. 2019;5(164):211–22. https://doi.org/10.1016/j.jpba.2018.10.046.
Shi Z, Hermiller J, Muñoz SG. Estimation of mass-based composition in powder mixtures using Extended Iterative Optimization Technology (EIOT). AIChE J. 2019;65(1):87–98. https://doi.org/10.1002/aic.16417.
Muñoz SG, Torres EH. Supervised extended iterative optimization technology for estimation of powder compositions in pharmaceutical applications: method and lifecycle management. Ind Eng Chem Res. 2020;59(21):10072–81. https://doi.org/10.1021/acs.iecr.0c01385
Rish AJ, Henson SR, AnikAlam M, Liu Y, Drennen JK, Anderson CA. Development of calibration-free/minimal calibration wavelength selection for iterative optimization technology algorithms toward process analytical technology application. Int J Pharm. 2022;614. https://doi.org/10.1016/j.ijpharm.2022.121463.
Román-Ospino AD, Baranwal Y, Li J, Vargas J, Igne B, Bate S, et al. Sampling optimization for blend monitoring of a low dose formulation in a tablet press feed frame using spatially resolved near-infrared spectroscopy. Int J Pharm. 2021;602. https://doi.org/10.1016/j.ijpharm.2021.120594.
Alam MA, Liu YA, Dolph S, Pawliczek M, Peeters E, Palm A. Benchtop NIR method development for continuous manufacturing scale to enable efficient PAT application for solid oral dosage form. Int J Pharm. 2021;601. https://doi.org/10.1016/j.ijpharm.2021.120581
Mendez R, Muzzio FJ, Velazquez C. Powder hydrophobicity and flow properties: Effect of feed frame design and operating parameters. AIChE J. 2012;58(3):697–706. https://doi.org/10.1002/aic.12639
Hernandez E, Pawar P, Keyvan G, Wang Y, Velez N, Callegari G, et al. Prediction of dissolution profiles by non-destructive near infrared spectroscopy in tablets subjected to different levels of strain. J Pharm Biomed Anal. 2016;117:568–76. https://doi.org/10.1016/j.ijpharm.2019.05.022.
Sierra-Vega NO, Karry KM, Romañach RJ, Méndez R. Monitoring of high-load dose formulations based on co-processed and non co-processed excipients. Int J Pharm. 2021;5(606). https://doi.org/10.1016/j.ijpharm.2021.120910.
USP-NF. General Chapter 〈1097〉 Bulk Powder Sampling Procedures. In: United States Pharmacopeia. 2023.
Sierra-Vega NO, Martínez-Cartagena PA, Alvarado-Hernández BB, Romañach RJ, Méndez R. In-line monitoring of low drug concentration of flowing powders in a new sampler device. Int J Pharm. 2020;583. https://doi.org/10.1016/j.ijpharm.2020.119358.
Llusa M, Levin M, Snee RD, Muzzio FJ. Measuring the hydrophobicity of lubricated blends of pharmaceutical excipients. Powder Technol. 2010;198(1):101–7. https://doi.org/10.1016/j.powtec.2009.10.021.
Mateo-Ortiz D, Colon Y, Romañach RJ, Méndez R. Analysis of powder phenomena inside a Fette 3090 feed frame using in-line NIR spectroscopy. J Pharm Biomed Anal. 2014;100:40–9. https://doi.org/10.1016/j.jpba.2014.07.014.
Dühlmeyer KP, Özcoban H, Leopold CS. A novel method for determination of the filling level in the feed frame of a rotary tablet press. Drug Dev Ind Pharm. 2018;44(11):1744–51. https://doi.org/10.1080/03639045.2018.1492609
Li Y, Anderson CA, Drennen JK, Airiau C, Igne B. Method Development and Validation of an Inline Process Analytical Technology Method for Blend Monitoring in the Tablet Feed Frame Using Raman Spectroscopy. Anal Chem. 2018;90(14):8436–44. https://doi.org/10.1021/acs.analchem.8b01009.
Esbensen KH, Román-Ospino AD, Sanchez A, Romañach RJ. Adequacy and verifiability of pharmaceutical mixtures and dose units by variographic analysis (Theory of Sampling) - A call for a regulatory paradigm shift. Int J Pharm. 2016;499(1–2):156–74. https://doi.org/10.1016/j.ijpharm.2015.12.038.
Alvarado-Hernández BB, Sierra-Vega NO, Martínez-Cartagena P, Hormaza M, Méndez R, Romañach RJ. A sampling system for flowing powders based on the theory of sampling. Int J Pharm. 2020;574. https://doi.org/10.1016/j.ijpharm.2019.118874.
Esbensen KH, Paoletti C, Minkkinen P. Representative sampling of large kernel lots I. Theory of Sampling and variographic analysis.Trends Anal Chem. 2012;32:154–64. https://doi.org/10.1016/j.trac.2011.09.008.
Esbensen KH, Julius LP. Representative sampling, data quality, validation - a necessary trinity in chemometrics. In: Brown SD, Tauler R and Walczak B (eds) Comprehensive Chemometrics. Oxford: Elsevier, 2009;1–20. https://doi.org/10.1016/B978-044452701-1.00088-0.
Sánchez-Paternina A, Sierra-Vega NO, Cárdenas V, Méndez R, Esbensen KH, Romañach RJ. Variographic analysis: A new methodology for quality assurance of pharmaceutical blending processes. Comput Chem Eng. 2019;124:109–23. https://doi.org/10.1016/j.compchemeng.2019.02.010.
EMA. ICH Q2 (R1): Validation of analytical procedures: text and methodology. Int Conf Harmon. 2005. Available from: https://www.ema.europa.eu/en/ich-q2r2-validation-analytical-procedures-scientific-guideline. Accessed 11 Jul 2023
EMA. Guideline on the use of Near Infrared Spectroscopy (NIRS) by the pharmaceutical industry and the data requirements for new submissions and variations. Eur Med Agency. 2014. Available from: https://www.ema.europa.eu/en/use-near-infrared-spectroscopy-nirs-pharmaceutical-industry-data-requirements-new-submissions. Accessed 11 Jul 2023.
FDA. Development and Submission of Near Infrared Analytical Procedures Guidance for Industry. Food Drug Adm. 2015. Available from: http://www.fda.gov/Drugs/GuidanceComplianceRegulatoryInformation/Guidances/default.htm. Accessed 08 Mar 2023.
Vargas JM, Roman-Ospino AD, Sanchez E, Romañach RJ. Evaluation of Analytical and Sampling Errors in the Prediction of the Active Pharmaceutical Ingredient Concentration in Blends From a Continuous Manufacturing Process. J Pharm Innov. 2017;12(2):155–67. https://doi.org/10.1007/s12247-017-9273-1.
Sierra-Vega NO, González-Rosario RA, Rangel-Gil RS, Romañach RJ, Méndez R. Quantitative analysis of blend uniformity within a Three-Chamber feed frame using simultaneously Raman and Near-Infrared spectroscopy. Int J Pharm. 2022;5(613). https://doi.org/10.1016/j.ijpharm.2021.121417.
Vargas JM, Nielsen S, Cárdenas V, Gonzalez A, Aymat EY, Almodovar E, et al. Process analytical technology in continuous manufacturing of a commercial pharmaceutical product. Int J Pharm. 2018;538(1–2):167–78. https://doi.org/10.1016/j.ijpharm.2018.01.003.
Acknowledgements
The authors greatly appreciate the assistance of Pedro Martínez-Cartagena and Raúl Rangel-Gil in the development of the experiments in the feed frame.
Funding
This research was funded by Puerto Rico Science Technology and Research Trust [grant number 2020–00116].
Author information
Authors and Affiliations
Contributions
Nathaly A. Movilla-Meza: conceptualization, methodology, data curation, software, investigation, writing original draft. Nobel O. Sierra-Vega: formal analysis and writing (review and editing). Bárbara B. Alvarado-Hernández: formal analysis and writing (review and editing). Rafael Méndez: conceptualization, methodology, review of drafts). Rodolfo J. Romañach: funding acquisition, project administration, resources, conceptualization, and review of draft manuscripts).
Corresponding author
Ethics declarations
Conflict of Interest
The authors declare that they have no known competing financial interests or personal relationships that could have appeared to influence the work reported in this paper.
Consent for Publication
All the authors read and approved the final manuscript.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Springer Nature or its licensor (e.g. a society or other partner) holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Movilla-Meza, N.A., Sierra-Vega, N.O., Alvarado-Hernández, B.B. et al. The Use of a Closed Feed Frame for the Development of Near-Infrared Spectroscopic Calibration Model to Determine Drug Concentration. Pharm Res 40, 2903–2916 (2023). https://doi.org/10.1007/s11095-023-03601-1
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11095-023-03601-1