Abstract
Background
Labetalol has an irreplaceable role in treating Hypertensive disorders of pregnancy (HDP), a common disease during pregnancy with a prevalence of 5.2–8.2%. However, there were big differences in dosage regimens between various guidelines.
Purpose
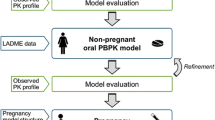
A physiologically-based pharmacokinetics (PBPK) model was established and validated to evaluate the existing oral dosage regimens, and to compare the difference in plasma concentration between pregnant and non-pregnant women.
Methods
First, non-pregnant woman models with specific plasma clearance or enzymatic metabolism (UGT1A1, UGT2B7, CYP2C19) were established and validated. For CYP2C19, slow, intermediate, and rapid metabolic phenotypes were considered. Then, a pregnant model with proper structure and parameters adjustment was established and validated against the multiple oral administration data.
Results
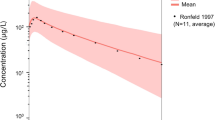
The predicted labetalol exposure captured the experimental data well. The following simulations with criteria lowering 15 mmHg blood pressure (corresponding to around 108 ng/ml plasma labetalol) found that the maximum daily dosage in the Chinese guideline may be insufficient for some severe HDP patients. Moreover, similar predicted steady-state trough plasma concentration was found between the maximum daily dosage in the American College of Obstetricians and Gynecologists (ACOG) guideline, 800 mg Q8h and a regimen of 200 mg Q6h. Simulations comparing non-pregnant and pregnant women found that the difference in labetalol exposure highly depended on the CYP2C19 metabolic phenotype.
Conclusions
In summary, this work initially established a PBPK model for multiple oral administration of labetalol for pregnant women. This PBPK model may lead to personalized labetalol medication in the future.



Similar content being viewed by others
References
Umesawa M, Kobashi G. Epidemiology of hypertensive disorders in pregnancy: prevalence, risk factors, predictors and prognosis. Hypertens Res. 2017;40(3):213–20.
Wilkerson RG, Ogunbodede AC. Hypertensive Disorders of Pregnancy. Emerg Med Clin North Am. 2019;37(2):301–16.
Li L, Fu QQ. Meta - analysis of prevalence of hypertensive disorder complicating pregnancy in China. Maternal and Child Health Care of China. 2019;34(14):3378–81.
Espinoza J, Vidaaeff A, Pettker CM, Simhan H. Gestational hypertension and preeclampsia: ACOG Practice Bulletin, number 222. Obstet Gynecol. 2020;135:e237–60.
Shimoyama T, Miller JP, Thompson GR. Preliminary studies of the pharmacological effects of 5-(1-HYDROXY-2-[(1-METHYL-3-PHENYLPROPYL)AMINO]-ETHYL)SALICYLAMIDE (AH 5158) IN MAN. Clin Sci. 1971;40(4):18P.
Pugsley D, Armstrong B, Nassim M, Beilin LJ. Combined alpha- and beta-adrenoreceptor blockade in hypertension: a controlled trial of labetalol (AH 5158) compared with propranolol and placebo. Clin Sci Mol Med Suppl. 1976;3(s3):501s–3s.
Lamming GD, Symonds EB. Use of labetalol and methyldopa in pregnancy-induced hypertension. Br J Clin Pharmacol. 1979;8 Suppl 2(S2):217S-222S.
WHO. WHO recommendations on drug treatment for non-severe hypertension in pregnancy: World Health Organization; 2020.
Yang Z, Zhang WY, Ling JH, Li XT, Liu JT, Qi H, et al. Guidelines for diagnosis and treatment of hypertensive disorder complicating pregnancy ( 2020 ). Chinese Journal of Practical Gynecololgy and Obstetrics. 2020;55(4):227–238.
Cifkova R, Johnson MR, Kahan T, Brguljan J, Williams B, Coca A, Manolis A, Thomopoulos C, Borghi C, Tsioufis C, Parati G, Sudano I, McManus RJ, van den Born BH, Regitz-Zagrosek V, de Simone G. Peripartum management of hypertension: a position paper of the ESC Council on Hypertension and the European Society of Hypertension. Eur Heart J Cardiovasc Pharmacother. 2020;6(6):384–93.
Zealand TWO-HN. Diagnosis and treatment of hypertension and pre-eclampsia in pregnancy in Aotearoa New Zealand. [cited 2022 October 20] Available from: https://www.tewhatuora.govt.nz/about-us/publications/diagnosis-and-treatment-of-hypertension-and-pre-eclampsia-in-pregnancy-in-aotearoa-new-zealand.
Butalia S, Audibert F, Côté A-M, Firoz T, Logan AG, Magee LA, Mundle W, Rey E, Rabi DM, Daskalopoulou SS. Hypertension Canada’s 2018 guidelines for the management of hypertension in pregnancy. Can J Cardiol. 2018;34(5):526–31.
Brown MA, Magee LA, Kenny LC, Karumanchi SA, McCarthy FP, Saito S, Hall DR, Warren CE, Adoyi G, Ishaku S. Hypertensive disorders of pregnancy: ISSHP classification, diagnosis, and management recommendations for international practice. Hypertension. 2018;72(1):24–43.
Guidelines QC. Hypertension and pregnancy. [cited 2021 May] Available from: https://www.health.qld.gov.au/__data/assets/pdf_file/0034/139948/g-hdp.pdf.
Mounier-Vehier C, Amar J, Boivin JM, Denolle T, Fauvel JP, Plu-Bureau G, Tsatsaris V, Blacher J. Hypertension and pregnancy: expert consensus statement from the French Society of Hypertension, an affiliate of the French Society of Cardiology. Fundam Clin Pharmacol. 2017;31(1):83–103.
Dawes M, Chowienczyk PJ. Drugs in pregnancy. Pharmacokinetics in pregnancy. Best Pract Res Clin Obstet Gynaecol. 2001;15(6):819–826.
Betcher HK, George Jr AL. Pharmacogenomics in pregnancy. In.Seminars in perinatology: Elsevier; 2020. p. 151222.
Ke AB, Rostami-Hodjegan A, Zhao P, Unadkat JD. Pharmacometrics in pregnancy: An unmet need. Annu Rev Pharmacol Toxicol. 2014;54(S2):53–69.
Chaphekar N, Caritis S, Venkataramanan R. Model-Informed Dose Optimization in Pregnancy. J Clin Pharmacol. 2020;60:S63–76.
FDA. Physiologically based pharmacokinetic analyses— format and content guidance for industry. [cited 2018 September] Available from: https://www.fda.gov/regulatory-information/search-fda-guidance-documents/physiologically-based-pharmacokinetic-analyses-format-and-content-guidance-industry.
Center for Drug evaluation N. Technical Guidelines for Drug-Drug Interaction Research (Draft for Comments). [cited 2018 September] Available from:https://www.cde.org.cn/main/news/viewInfoCommon/97372c503b10b905f36c256bcb471edb.
Dallmann A, Solodenko J, Ince I, Eissing T. Applied concepts in PBPK modeling: How to extend an open systems pharmacology model to the special population of pregnant women. CPT Pharmacometrics Syst Pharmacol. 2018;7(7):419–31.
Zur M, Gasparini M, Wolk O, Amidon GL, Dahan A. The low/high BCS permeability class boundary: physicochemical comparison of metoprolol and labetalol. Mol Pharm. 2014;11(5):1707–14.
Plachka K, Svec F, Novakova L. Ultra-high performance supercritical fluid chromatography in impurity control: Searching for generic screening approach. Anal Chim Acta. 2018;1039:149–61.
Hermann KF, Neuhaus CS, Micallef V, Wagner B, Hatibovic M, Aschmann HE, Paech F, Alvarez-Sanchez R, Kramer SD, Belli S. Kinetics of lipid bilayer permeation of a series of ionisable drugs and their correlation with human transporter-independent intestinal permeability. Eur J Pharm Sci. 2017;104:150–61.
Daneshmend T, Roberts C. The influence of food on the oral and intravenous pharmacokinetics of a high clearance drug: a study with labetalol. Br J Clin Pharmacol. 1982;14(1):73–8.
Daneshmend TK, Roberts CJ. The effects of enzyme induction and enzyme inhibition on labetalol pharmacokinetics. Br J Clin Pharmacol. 1984;18(3):393–400.
Lalonde RL, O’Rear TL, Wainer IW, Drda KD, Herring VL, Bottorff MB. Labetalol pharmacokinetics and pharmacodynamics: evidence of stereoselective disposition. Clin Pharmacol Ther. 1990;48(5):509–19.
Tran NL. Bioequivealency review(s): Center for Drug evaluation and Research ANDA75133. [cited 1998 March 8] Available from:https://www.accessdata.fda.gov/scripts/cder/daf/index.cfm?event=overview.process&ApplNo=075133.
Jeong H, Choi S, Song JW, Chen H, Fischer JH. Regulation of UDP-glucuronosyltransferase (UGT) 1A1 by progesterone and its impact on labetalol elimination. Xenobiotica. 2008;38(1):62–75.
Chan SW, Hu M, Ko SS, Tam CW, Fok BS, Yin OQ, Chow MS, Tomlinson B. CYP2C19 genotype has a major influence on labetalol pharmacokinetics in healthy male Chinese subjects. Eur J Clin Pharmacol. 2013;69(4):799–806.
Dallmann A, Ince I, Coboeken K, Eissing T, Hempel G. A Physiologically Based Pharmacokinetic Model for Pregnant Women to Predict the Pharmacokinetics of Drugs Metabolized Via Several Enzymatic Pathways. Clin Pharmacokinet. 2018;57(6):749–68.
Saotome T, Minoura S, Terashi K, Sato T, Echizen H, Ishizaki T. Labetalol in hypertension during the third trimester of pregnancy: its antihypertensive effect and pharmacokinetic-dynamic analysis. J Clin Pharmacol. 1993;33(10):979–88.
Wang W, Ouyang D. Prediction of Free Drug Absorption in Cyclodextrin Formulation by a Modified Physiologically Based Pharmacokinetic Model and Phase Solubility 3-D Surface Graph. Pharm Res. 2021;38(7):1157–68.
Choi SJH, Deyo K, Fischer J. Protein binding of labetalol in pregnancy. Clinical and Pharmacological Therapy. 2007;81(S1):S79.
McNeil JJ, Anderson AE, Louis WJ. Labetalol steady-state pharmacokinetics in hypertensive patients. Br J Clin Pharmacol. 1982;13(1 Suppl):75S-80S.
Hopkins R, Martin LE, Bland R. The metabolism of labetalol in animals and man. Biochem Soc Trans. 1976;4(4):726–9.
Mantyla R, Allonen H, Kanto J, Kleimola T, Sellman R. Effect of food on the bioavailability of labetalol. Br J Clin Pharmacol. 1980;9(4):435–7.
Maronde RF, Robinson D, Vlachakis ND, Barr JW, Chung M, Zampaglione N, Medakovic M. Study of single and multiple dose pharmacokinetic/pharmacodynamic modeling of the antihypertensive effects of labetalol. Am J Med. 1983;75(4A):40–6.
McNeil JJ, Anderson AE, Louis WJ, Morgan DJ. Pharmacokinetics and pharmacodynamic studies of labetalol in hypertensive subjects. Br J Clin Pharmacol. 1979;8 Suppl 2(2):157S-161S.
Rocci M L VPH, MD Cressman, et al. Pharmacokinetics and pharmacodynamics of labetalol in elderly and young hypertensive patients following single and multiple doses. Pharmacotherapy. 1990.
Daneshmend T, Roberts C. The short term effects of propranolol, atenolol and labetalol on antipyrine kinetics in normal subjects. Br J Clin Pharmacol. 1982;13(6):817–20.
Hermann DJ, Krol TF, Dukes GE, Hussey EK, Danis M, Han YH, Powell JR, Hak LJ. Comparison of Verapamil, Diltiazem, and Labetalol on the Bioavailability and Metabolism of Imipramine. J Clin Pharmacol. 1992;32(2):176–83.
Inc PL. TRANDATE®(labetalol hydrochloride) Tablets product information. [cited 2010 November 22] Available from: https://www.accessdata.fda.gov/drugsatfda_docs/label/2010/018716s026lbl.pdf.
Rubin PC, Butters L, Kelman AW, Fitzsimons C, Reid JL. Labetalol disposition and concentration-effect relationships during pregnancy. Br J Clin Pharmacol. 1983;15(4):465–70.
McNeil JJ, Louis WJ. Clinical pharmacokinetics of labetalol. Clin Pharmacokinet. 1984;9(2):157–67.
Abushammala I, Garrigues TM, Casabo VG, Nacher A, Martin-Villodre A. Labetalol absorption kinetics: rat small intestine and colon studies. J Pharm Sci. 2006;95(8):1733–41.
Hebert MF, Easterling TR, Kirby B, Carr DB, Buchanan ML, Rutherford T, Thummel KE, Fishbein DP, Unadkat JD. Effects of pregnancy on CYP3A and P-glycoprotein activities as measured by disposition of midazolam and digoxin: a University of Washington specialized center of research study. Clin Pharmacol Ther. 2008;84(2):248–53.
Funding
Current research is financially supported by the Macau FDCT Research Grant (0108/2021/A) and the University of Macau Research Grant (MYRG2020-00113-ICMS).
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of Interest
The authors report no conflicts of interest.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary Information
Below is the link to the electronic supplementary material.
Rights and permissions
Springer Nature or its licensor (e.g. a society or other partner) holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Song, Y., Wang, W., Liu, X. et al. Physiologically Based Pharmacokinetic Modeling for Multiple Oral Administration Labetalol in Pregnant Women. Pharm Res 40, 1765–1775 (2023). https://doi.org/10.1007/s11095-023-03523-y
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11095-023-03523-y