Abstract
Purpose
Vancomycin-resistant enterococci (VRE) have recently become a major cause of nosocomial infections and a global public health concern. Tedizolid exhibits powerful antibacterial activity against VRE in vitro, but its pharmacokinetic/pharmacodynamic (PK/PD) parameters remain unclear. Therefore, we aimed to determine the PK/PD indices of tedizolid action on VRE and the mechanisms underlying the PK/PD indices differences of tedizolid against VRE and methicillin-resistant Staphylococcus aureus (MRSA).
Methods
Optimal PK/PD target values of tedizolid were determined in vitro, based on time-kill curves and post-antibiotic effects (PAEs), and in vivo, using mouse models of thigh infection with VRE and MRSA strains.
Results
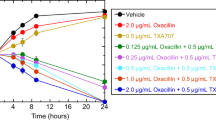
The tedizolid bactericidal activity on VRE and MRSA was time-dependent. Correlations were closest between fAUC24/MIC and the tedizolid PK/PD index against MRSA and VRE. To achieve 1 log10 kill tedizolid fAUC24/MIC in neutropenic mouse models of thigh infection with VRE and MRSA should be 14.2 and 138.5, respectively. The PAEs of tedizolid against VRE and MRSA were 2.39 and 0.99 h, respectively.
Conclusion
Tedizolid showed bactericidal effects against VRE even in neutropenic mice unlike MRSA, which could be attributed to its longer PAE against VRE. Hence, we hypothesize that tedizolid treatment against VRE infections is promising for achieving therapeutic success in clinical.






Similar content being viewed by others
Data Availability
The dataset generated or analyzed during the current study is available from the corresponding author up reasonable request.
References
Shrestha S, Kharel S, Homagain S, Aryal R, Mishra SK. Prevalence of vancomycin‐resistant enterococci in Asia—A systematic review and meta-analysis. J Clin Pharm Ther. 2021;46:1226–37. https://onlinelibrary.wiley.com/doi/10.1111/jcpt.13383.
Lebreton F, Willems RJL, Gilmore MS, et al. Enterococcus diversity, origins in nature, and gut colonization; 2014. https://www.ncbi.nlm.nih.gov/books/NBK190427/. Boston: Massachusetts Eye and Ear Infirmary.
Cetinkaya Y, Falk P, Mayhall CG. Vancomycin-resistant enterococci. Clin Microbiol Rev. 2000;13:686–707. http://cmr.asm.org/cgi/doi/10.1128/CMR.13.4.686-707.2000.
Zirakzadeh A, Patel R. Vancomycin-resistant enterococci: Colonization, infection, detection, and treatment. Mayo Clin Proc. 2006;81:529–36. https://linkinghub.elsevier.com/retrieve/pii/S0025619611619010. https://doi.org/10.4065/81.4.529
Sandison T, De Anda C, Fang E, Das AF, Prokocimer P. Clinical Response of tedizolid versus linezolid in Acute Bacterial Skin and Skin Structure Infections by Severity Measure Using a Pooled Analysis from Two Phase 3 Double-Blind Trials. Antimicrob Agents Chemother. 2017;61. https://journals.asm.org/doi/10.1128/AAC.02687-16.
Lodise TP, Fang E, Minassian SL, Prokocimer PG. Platelet profile in patients with acute bacterial skin and skin structure infections receiving tedizolid or linezolid: Findings from the Phase 3 ESTABLISH clinical trials. Antimicrob Agents Chemother. 2014;58:7198–204. https://journals.asm.org/doi/10.1128/AAC.03509-14.
Wang Y, Lv Y, Cai J, Schwarz S, Cui L, Hu Z, et al. A novel gene, optrA, that confers transferable resistance to oxazolidinones and phenicols and its presence in Enterococcus faecalis and Enterococcus faecium of human and animal origin. J Antimicrob Chemother. 2015;70:2182–90. https://academic.oup.com/jac/article/70/8/2182/812642. https://doi.org/10.1093/jac/dkv116
Sahm DF, Deane J, Bien PA, Locke JB, Zuill DE, Shaw KJ, et al. Results of the surveillance of tedizolid activity and resistance program: In vitro susceptibility of gram-positive pathogens collected in 2011 and 2012 from the United States and Europe. Diagn Microbiol Infect Dis. 2015;81:112–8. https://doi.org/10.1016/j.diagmicrobio.2014.08.011.
Drusano GL, Liu W, Kulawy R, Louie A. Impact of granulocytes on the antimicrobial effect of tedizolid in a mouse thigh infection model. Antimicrob Agents Chemother. 2011;55:5300–5. https://doi.org/10.1128/AAC.00502-11.
Clinical and Laboratory Standards Institute. Methods for dilution antimicrobial susceptibility tests for bacteria that grow aerobically; approved standard—Tenth edition: M07-A10. Wayne, PA: NCCLS; 2015
Clinical and Laboratory Standards Institute. Performance Standards for antimicrobial Susceptibility Testing—Twenty-seventh edition: M100. Wayne, PA: NCCLS; 2017
Clinical and Laboratory Standards Institute. Methods for determining bactericidal activity of antimicrobial agents;approved guideline: M26-A. Wayne, PA: NCCLS; 1999
Yokoyama Y, Matsumoto K, Ikawa K, Watanabe E, Shigemi A, Umezaki Y, et al. Pharmacokinetic/pharmacodynamic evaluation of sulbactam against Acinetobacter baumannii in in vitro and murine thigh and lung infection models. Int J Antimicrob Agents. 2014;43:547–52. https://linkinghub.elsevier.com/retrieve/pii/S0924857914000831. https://doi.org/10.1016/j.ijantimicag.2014.02.012
Zuluaga AF, Salazar BE, Rodriguez CA, Zapata AX, Agudelo M, Vesga O. Neutropenia induced in outbred mice by a simplified low-dose cyclophosphamide regimen: Characterization and applicability to diverse experimental models of infectious diseases. BMC Infect Dis. 2006;6:55. https://bmcinfectdis.biomedcentral.com/articles/10.1186/1471-2334-6-55.
Ong V, Flanagan S, Fang E, Dreskin HJ, Locke JB, Bartizal K, et al. Absorption, distribution, metabolism, and excretion of the novel antibacterial prodrug tedizolid phosphate. Drug Metab Dispos. 2014;42(8):1275–84.
Craig WA. The postantibiotic effect. Clin Microbiol Newsl. 1991;13:121–4. https://linkinghub.elsevier.com/retrieve/pii/019643999190030Y.
Boswell FJ, Andrews JM, Wise R. Pharmacodynamic properties of faropenem demonstrated by studies of 1997. p. 415–8
Abdelhady W, Mishra NN. Comparative Efficacies of linezolid vs. tedizolid in an Experimental Murine Model of Vancomycin-Resistant Enterococcal (VRE) bacteremia. Front Med (Lausanne). 2019;6. https://www.frontiersin.org/article/10.3389/fmed.2019.00031/full:31.
Wang S, Liu H, Mao J, Peng Y, Yan Y, Li Y, et al. Pharmacodynamics of linezolid plus fosfomycin against vancomycin–resistant Enterococcus faecium in a hollow fiber infection model. Front Microbiol. 2021;12. https://www.frontiersin.org/articles/10.3389/fmicb.2021.779885/full:779885.
McKay GA, Beaulieu S, Arhin FF, Belley A, Sarmiento I, Parr T, et al. Time-kill kinetics of oritavancin and comparator agents against Staphylococcus aureus, Enterococcus faecalis and Enterococcus faecium. J Antimicrob Chemother. 2009;63:1191–9. https://academic.oup.com/jac/article-lookup/doi/10.1093/jac/dkp126.
Larsson AJ, Walker KJ, Raddatz JK, Rotschafer JC. The concentration-independent effect of monoexponential and biexponential decay in vancomycin concentrations on the killing of Staphylococcus aureus under aerobic and anaerobic conditions. J Antimicrob Chemother. 1996;38:589–97. https://academic.oup.com/jac/article-lookup/doi/10.1093/jac/38.4.589.
Rybak MJ. The pharmacokinetic and pharmacodynamic properties of vancomycin. Clin Infect Dis. 2006;42;Suppl 1(Supplement_1):S35–9. http://academic.oup.com/cid/article/42/Supplement_1/S35/275535/The-Pharmacokinetic-and-Pharmacodynamic-Properties.
Takemura W, Tashiro S, Hayashi M, Igarashi Y, Liu X, Mizukami Y, et al. Cefmetazole as an alternative to carbapenems against extended-spectrum beta-lactamase-producing Escherichia coli infections based on in vitro and in vivo pharmacokinetics/pharmacodynamics experiments. Pharm Res. 2021;38:1839–46. https://link.springer.com/10.1007/s11095-021-03140-7.
Fuentes F, Izquierdo J, Martín MM, Gomez-Lus ML, Prieto J. Postantibiotic and sub-MIC effects of azithromycin and isepamicin against Staphylococcus aureus and Escherichia coli. Vol. 42, Antimicrobial Agents and Chemotherapy. 1998. p. 414–8.
Li RC, Zhu ZY. The integration of four major determinants of Antibiotic Action: Bactericidal activity, postantibiotic effect, susceptibility, and pharmacokinetics. J Chemother. 2002;14:579–83. http://www.tandfonline.com/doi/full/10.1179/joc.2002.14.6.579.
Carvalhaes CG, Sader HS, Flamm RK, Streit JM, Mendes RE. Assessment of tedizolid in vitro activity and resistance mechanisms against a collection of Enterococcus spp. causing invasive infections, including isolates requiring an optimized dosing strategy for daptomycin from U.S. and European Medical Centers, 2016 to 2018. Antimicrob Agents Chemother. 2020;64:2016–8. https://doi.org/10.1128/AAC.00175-20
Ambrose PG, Bhavnani SM, Rubino CM, Louie A, Gumbo T, Forrest A, et al. Antimicrobial resistance: Pharmacokinetics‐pharmacodynamics of Antimicrobial Therapy: It’s not just for mice anymore. Clin Infect Dis. 2007;44:79–86. https://academic.oup.com/cid/article-lookup/doi/ 10.1086/510079.
Chen R, Shen K, Chang X, Tanaka T, Li L, Hu P. Pharmacokinetics and safety of tedizolid after single and multiple intravenous/oral sequential administrations in healthy Chinese subjects. Clin Ther. 2016;38:1869–79. https://linkinghub.elsevier.com/retrieve/pii/S0149291816304295. https://doi.org/10.1016/j.clinthera.2016.06.014
Kim Y, Kim A, Lee S, Choi SH, Lee DY, Song JS, et al. Pharmacokinetics, safety, and tolerability of tedizolid phosphate after single-dose administration in healthy Korean male subjects. Clin Ther. 2017;39:1849–57. https://linkinghub.elsevier.com/retrieve/pii/S0149291817308731. https://doi.org/10.1016/j.clinthera.2017.08.002
Patterson JE, Sweeney AH, Simms M, Carley N, Mangi R, Sabetta J, et al. An analysis of 110 enterococcal infections epidemiology, antibiotic susceptibility, and outcome. Med (Baltim). 1995;74:191–200. http://journals.lww.com/00005792-199507000-00003. https://doi.org/10.1097/00005792-199507000-00003
Xiao J, Gill C, Liang L, Liu J, Wu J, Tan CM, et al. Use of translational PKPD infection models to understand impact of neutropenia on efficacy of tedizolid phosphate. Open Forum Infect Dis. 2017;4(suppl_1):S292–S292. https://doi.org/10.1093/ofid/ofx163.667
Acknowledgements
We thank Editage (www.editage.com) for the English language editing.
Author information
Authors and Affiliations
Contributions
Conceptualization: Xiaoxi Liu, Sho Tashiro, Yuki Igarashi, Wataru Takemura, Nana Kojima, Takumi Morita, Marina Hayashi, Yuki Enoki, Kazuaki Taguchi and Kazuaki Matsumoto.
Data curation: Xiaoxi Liu, Yuki Enoki, Kazuaki Taguchi, Kazuaki Matsumoto.
Formal analysis: Xiaoxi Liu, Yuki Enoki.
Investigation: Xiaoxi Liu, Yuki Enoki, Kazuaki Taguchi, Kazuaki Matsumoto. Methodology: Xiaoxi Liu, Sho Tashiro, Yuki Igarashi, Wataru Takemura, Nana Kojima, Takumi Morita, and Marina Hayashi.
Project administration: Xiaoxi Liu, Yuki Enoki, Kazuaki Taguchi, and Kazuaki Matsumoto. Resources: Yuki Enoki, Kazuaki Taguchi, and Kazuaki Matsumoto.
Supervision: Yuki Enoki, Kazuaki Taguchi, Kazuaki Matsumoto.
Validation: Sho Tashiro, Yuki Igarashi, Wataru Takemura, Nana Kojima, Takumi Morita, and Marina Hayashi.
Visualization: Sho Tashiro, Yuki Igarashi, Wataru Takemura, Nana Kojima, Takumi Morita, and Marina Hayashi.
Writing-original draft: Xiaoxi Liu.
Writing-review and editing: Yuki Enoki, Kazuaki Matsumoto.
Corresponding author
Ethics declarations
Conflict of Interest Statement
The authors report no conflicts of interest.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary Information
Below is the link to the electronic supplementary material.
Rights and permissions
Springer Nature or its licensor (e.g. a society or other partner) holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Liu, X., Tashiro, S., Igarashi, Y. et al. Differences in Pharmacokinetic/Pharmacodynamic Parameters of Tedizolid Against VRE and MRSA. Pharm Res 40, 187–196 (2023). https://doi.org/10.1007/s11095-022-03425-5
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11095-022-03425-5