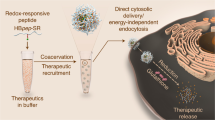
Abstract
Purpose
Cytosolic delivery of proteins accesses intracellular targets for chemotherapy and immunomodulation. Current delivery systems utilize inefficient endosomal pathways of uptake and escape that lead to degradation of delivered cargo. Cationic poly(oxanorbornene)imide (PONI) polymers enable highly efficient cytosolic delivery of co-engineered proteins, but aggregation and denaturation in solution limits shelf life. In the present study we evaluate polymer-protein nanocomposite vehicles as candidates for lyophilization and point-of-care resuspension to provide a transferrable technology for cytosolic protein delivery.
Methods
Self-assembled nanocomposites of engineered poly(glutamate)-tagged (E-tagged) proteins and guanidinium-functionalized PONI homopolymers were generated, lyophilized, and stored for 2 weeks. After reconstitution and delivery, cytosolic access of E-tagged GFP cargo (GFPE15) was assessed through diffuse cytosolic and nuclear fluorescence, and cell killing with chemotherapeutic enzyme Granzyme A (GrAE10). Efficiency was quantified between freshly prepared and lyophilized samples.
Results
Reconstituted nanocomposites retained key structural features of freshly prepared assemblies, with minimal loss of material. Cytosolic delivery (> 80% efficiency of freshly prepared nanocomposites) of GFPE15 was validated in several cell lines, with intracellular access validated and quantified through diffusion into the nucleus. Delivery of GrAE10 elicited significant tumorigenic cell death. Intracellular access of cytotoxic protein was validated through cell viability.
Conclusion
Reconstituted nanocomposites achieved efficient cytosolic delivery of protein cargo and demonstrated therapeutic applicability with delivery of GrAE10. Overall, this strategy represents a versatile and highly translatable method for cytosolic delivery of proteins.
Graphical Abstract





Similar content being viewed by others
Abbreviations
- DLS:
-
Dynamic light scattering
- DMEM:
-
Dulbecco’s Modified Eagle Medium
- DPBS:
-
Dulbecco’s phosphate-buffered saline
- EDTA:
-
Ethylenediaminetetraacetic acid
- FBS:
-
Fetal bovine serum
- GFP:
-
Green fluorescent protein
- MFI:
-
Mean fluorescence intensity
- MW:
-
Molecular weight
- PONI:
-
Poly(oxanorbornene)imide
- TCEP:
-
Tris(2-carboxyethyl)phosphine
References
Vargason AM, Anselmo AC, Mitragotri S. The evolution of commercial drug delivery technologies. Nat Biomed Eng. 2021;5:951–67.
Scaletti F, Hardie J, Lee Y-W, Luther DC, Ray M, Rotello VM. Protein delivery into cells using inorganic nanoparticle-protein supramolecular assemblies. Chem Soc Rev. 2018;47:3421–32.
Luther DC, Jeon T, Goswami R, Nagaraj H, Kim D, Lee YW, et al. Protein delivery: if your GFP (or other small protein) is in the cytosol, it will also be in the nucleus. Bioconjug Chem. 2021;32:891–6.
Luther DC, Huang R, Jeon T, Zhang X, Lee Y-W, Nagaraj H, et al. Delivery of drugs, proteins, and nucleic acids using inorganic nanoparticles. Adv Drug Deliv Rev. 2020;156:188–213.
Teo SLY, Rennick JJ, Yuen D, Al-Wassiti H, Johnston APR, Pouton CW. Unravelling cytosolic delivery of cell penetrating peptides with a quantitative endosomal escape assay. Nat Commun. 2021;12.
Smith SA, Selby LI, Johnston APR, Such GK. The endosomal escape of nanoparticles: toward more efficient cellular delivery. Bioconjug Chem. 2019;30:263–72.
Schneider AFL, Wallabregue ALD, Franz L, Hackenberger CPR. Targeted subcellular protein delivery using cleavable cyclic cell-penetrating peptides. Bioconjug Chem. 2019;30:400–4.
Chen YP, Chen CT, Hung Y, Chou CM, Liu TP, Liang MR, et al. A new strategy for intracellular delivery of enzyme using mesoporous silica nanoparticles: superoxide dismutase. J Am Chem Soc. 2013;135:1516–23.
Vazdar M, Heyda J, Mason PE, Tesei G, Allolio C, Lund M, et al. Arginine “magic”: guanidinium like-charge ion pairing from aqueous salts to cell penetrating peptides. Acc Chem Res. 2018;51:1455–64.
Cho EY, Ryu JY, Lee HAR, Hong SH, Park HS, Hong KS, et al. Lecithin nano-liposomal particle as a CRISPR/Cas9 complex delivery system for treating type 2 diabetes. J Nanobiotechnol. 2019;17:1–12.
Filipczak N, Pan J, Yalamarty SSK, Torchilin VP. Recent advancements in liposome technology. Adv Drug Deliv Rev. 2020;156:4–22.
Jiang Y, Hardie J, Liu Y, Ray M, Luo X, Das R, et al. Nanocapsule-mediated cytosolic siRNA delivery for anti-inflammatory treatment. J Control Release. 2018;283:235–40.
Lee YW, Luther DC, Goswami R, Jeon T, Clark V, Elia J, et al. Direct cytosolic delivery of proteins through coengineering of proteins and polymeric delivery vehicles. J Am Chem Soc. 2020;142:4349–55.
Chi EY, Krishnan S, Randolph TW, Carpenter JF. Physical stability of proteins in aqueous solution: mechanism and driving forces in nonnative protein aggregation. Pharm Res. 2003;20:1325–36.
Roberts CJ. Therapeutic protein aggregation: mechanisms, design, and control. Trends Biotechnol. 2014;32:372–80.
Weiss WF IV, Young TM, Roberts CJ. Principles, approaches, and challenges for predicting protein aggregation rates and shelf life. J Pharm Sci. 2009;98:1246–77.
Wang W. Instability, stabilization, and formulation of liquid protein pharmaceuticals. Int J Pharm. 1999;185:129–88.
Rajan R, Ahmed S, Sharma N, Kumar N, Debas A, Matsumura K. Review of the current state of protein aggregation inhibition from a materials chemistry perspective: special focus on polymeric materials. Mater Adv. 2021;2:1139–76.
Sofronova AA, Izumrudov VA, Muronetz VI, Semenyuk PI. Similarly charged polyelectrolyte can be the most efficient suppressor of the protein aggregation. Polymer. 2017;108:281–7.
Debnath K, Shekhar S, Kumar V, Jana NR. Efficient inhibition of protein aggregation, disintegration of aggregates, and lowering of cytotoxicity by green tea polyphenol-based self-assembled polymer nanoparticles. ACS Appl Mater Interfaces. 2016;8:20309–18.
Fonte P, Reis S, Sarmento B. Facts and evidences on the lyophilization of polymeric nanoparticles for drug delivery. J Control Release. 2016;225:75–86.
Lemoine D, Francois C, Kedzierewicz F, Preat V, Hoffman M, Maincent P. Stability study of nanoparticles of and poly (D, L-lactide-co-glycolide ). Biomaterials. 1996;17:2191–7.
Emami F, Vatanara A, Park EJ, Na DH. Drying technologies for the stability and bioavailability of biopharmaceuticals. Pharmaceutics. 2018;10:1–22.
Mensink MA, Frijlink HW, van der Voort MK, Hinrichs WLJ. How sugars protect proteins in the solid state and during drying (review): mechanisms of stabilization in relation to stress conditions. Eur J Pharm Biopharm. 2017;114:288–95.
Preston KB, Randolph TW. Stability of lyophilized and spray dried vaccine formulations. Adv Drug Deliv Rev. 2021;171:50–61.
Stärtzel P. Arginine as an excipient for protein freeze-drying: a mini review. J Pharm Sci. 2018;107:960–7.
Mukalel AJ, Evans BC, Kilchrist KV, Dailing EA, Burdette B, Cheung-Flynn J, et al. Excipients for the lyoprotection of MAPKAP kinase 2 inhibitory peptide nano-polyplexes. J Control Release. 2018;282:110–9.
Kim KR, Ahn KY, Park JS, Lee KE, Jeon H, Lee J. Lyophilization and enhanced stability of fluorescent protein nanoparticles. Biochem Biophys Res Commun. 2011;408:225–9.
Mason PE, Neilson GW, Dempsey CE, Barnes AC, Cruickshank JM. The hydration structure of guanidinium and thiocyanate ions: Implications for protein stability in aqueous solution. Proc Natl Acad Sci. 2003;100:4557–61.
Peveler WJ, Landis RF, Yazdani M, Day JW, Modi R, Carmalt CJ, Rosenberg WM, Rotello VM. A rapid and robust diagnostic for liver fibrosis using a multichannel polymer sensor array. Adv Mater. 2018;30:1800634.
Ngernpimai S, Geng Y, Makabenta JM, Landis RF, Keshri P, Gupta A, Li C-H, Chompoosor A, Rotello VM. Rapid identification of biofilms using a robust multichannel polymer sensor array. ACS Appl Mater Interfaces. 2019;11:11202–8.
Mout R, Ray M, Tay T, Sasaki K, Yesilbag Tonga G, Rotello VM. General strategy for direct cytosolic protein delivery via protein–nanoparticle co-engineering. ACS Nano. 2017;11:6416–21.
Remmele RL Jr, Stushnoff C, Carpenter JF. Real-time in situ monitoring of lysozyme during lyophilization using infrared spectroscopy: dehydration stress in the presence of sucrose. Pharm Res. 1997;14:1548–55.
Simpson RJ. Stabilization of proteins for storage. Cold Spring Harb Protoc. 2010;5:79–120.
Ó’Fágáin C, Colliton K. Storage and lyophilization of pure proteins. Methods Mol Biol. 2017;1485:159–90.
Yoshioka S, Miyazaki T, Aso Y, Kawanishi T. Significance of local mobility in aggregation of β-galactosidase lyophilized with trehalose, sucrose or stachyose. Pharm Res. 2007;24:1660–7.
Billen B, Vincke C, Hansen R, Devoogdt N, Muyldermans S, Adriaensens P, et al. Cytoplasmic versus periplasmic expression of site-specifically and bioorthogonally functionalized nanobodies using expressed protein ligation. Protein Expr Purif. 2017;133:25–34.
Dong C, Garen CR, Mercier P, Petersen NO, Woodside MT. Characterizing the inhibition of α-synuclein oligomerization by a pharmacological chaperone that prevents prion formation by the protein PrP. Protein Sci. 2019;28:1690–702.
Saeed IA, Ashraf SS. Denaturation studies reveal significant differences between GFP and blue fluorescent protein. Int J Biol Macromol. 2009;45:236–41.
Bhatnagar BS, Bogner RH, Pikal MJ. Protein stability during freezing: separation of stresses and mechanisms of protein stabilization. Pharm Dev Technol. 2007;12:505–23.
Roughton BC, Iyer LK, Bertelsen E, Topp EM, Camarda KV. Protein aggregation and lyophilization: Protein structural descriptors as predictors of aggregation propensity. Comput Chem Eng. 2013;58:369–77.
Zhang S, Lv J, Gao P, Feng Q, Wang H, Cheng Y. A pH-responsive phase-transition polymer with high serum stability in cytosolic protein delivery. Nano Lett. 2021;21:7855–61.
Schneider AFL, Wallabregue ALD, Franz L, Hackenberger CPR. Targeted subcellular protein delivery using cleavable cyclic cell-penetrating peptides. Bioconjug Chem. 2019;30:400–4.
Allolio C, Magarkar A, Jurkiewicz P, Baxová K, Javanainen M, Mason PE, et al. Arginine-rich cell-penetrating peptides induce membrane multilamellarity and subsequently enter via formation of a fusion pore. Proc Natl Acad Sci. 2018;115:11923–8.
Monnery BD. Polycation-mediated transfection: Mechanisms of internalization and intracellular trafficking. Biomacromol. 2021;22:4060–83.
van Daalen KR, Reijneveld JF, Bovenschen N. Modulation of inflammation by extracellular granzyme A. Front Immunol. 2020;11:1–11.
Hink-Schauer C, Estébanez-Perpiñá E, Kurschus FC, Bode W, Jenne DE. Crystal structure of the apoptosis-inducing human granzyme a dimer. Nat Struct Biol. 2003;10:535–40.
Bots M, Medema JP. Granzymes at a glance. J Cell Sci. 2006;119:5011–4.
Zhou Z, He H, Wang K, Shi X, Wang Y, Su Y, et al. Granzyme A from cytotoxic lymphocytes cleaves GSDMB to trigger pyroptosis in target cells. Science. 2020;368:7548.
Grodzovski I, Lichtenstein M, Galski H, Lorberboum-Galski H. IL-2-granzyme A chimeric protein overcomes multidrug resistance (MDR) through a caspase 3-independent apoptotic pathway. Int J Cancer. 2011;128:1966–80.
Garzón-Tituaña M, Sierra-Monzón JL, Comas L, Santiago L, Khaliulina-Ushakova T, Uranga-Murillo I, et al. Granzyme A inhibition reduces inflammation and increases survival during abdominal sepsis. Theranostics. 2021;11:3781–95.
ACKNOWLEDGMENTS AND DISCLOSURE
The authors acknowledge Dr. James Chambers and the Light Microscopy Core Facility, and Dr. Amy Burnside and the Flow Cytometry Core Facility at UMass Amherst. The authors declare no competing financial interests.
Funding
This research was supported by the NIH (EB022641 and DK121351).
Author information
Authors and Affiliations
Corresponding author
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary Information
Below is the link to the electronic supplementary material.
Rights and permissions
About this article
Cite this article
Luther, D.C., Nagaraj, H., Goswami, R. et al. Direct Cytosolic Delivery of Proteins Using Lyophilized and Reconstituted Polymer-Protein Assemblies. Pharm Res 39, 1197–1204 (2022). https://doi.org/10.1007/s11095-022-03226-w
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11095-022-03226-w