Abstract
Purpose
Methicillin-resistant Staphylococcus aureus (MRSA) infection at impaired wound is associated with high risks of developing to persistent bacterial infections since bacterial biofilm is easy to form in MRSA infected wounds. An advanced therapeutic approach to effectively penetrate and eliminate bacterial biofilm and to accelerate cell proliferation and migration at the wound is crucial.
Methods
The poly(ε-caprolactone)-monomethoxyl poly (ethylene glycol) (PCL-mPEG) micelles loaded with Quercetin and Rifampicin (QRMs) were prepared. Bacterial biofilm proliferation and elimination effect of QRMs were evaluated with confocal laser scanning microscopy. Antibacterial assay was further performed to detect antibacterial activity and mechanism. The cell scratch assay and cellular uptake were performed in HaCaT skin epithelial cells.
Results
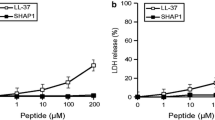
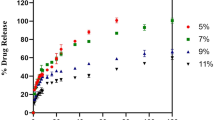
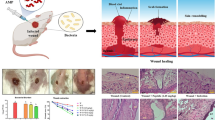
Our results showed that the small sized QRMs could penetrate the interior of MRSA biofilm to disperse and eradicate biofilm. Then, antibiotics are released and accumulated in the acidic biofilm environment. QRMs could kill bacteria through increasing bacterial membrane permeability and altering membrane potential and membrane fluidity. Moreover, QRMs improved intracellular and cytoplasmic delivery efficiency of drugs to epithelial cells, and in the scratch test, presented a stronger ability to promote migration and proliferation of HaCaT cells compared with free drugs. Hemolysis test further proved good biocompatibility of QRMs.
Conclusions
QRMs could potentially be used as a novel dual-functional nanotherapeutic for anti-bacterial infection by eradicating biofilm and accelerating cells proliferation at MRSA infected wound.






Similar content being viewed by others
Abbreviations
- C6:
-
Coumarin 6
- CMs:
-
Coumarin 6 loaded micelles
- CV:
-
Crystal violet
- DLS:
-
Dynamic light scattering spectroscopy
- EM:
-
Empty micelles
- EPS:
-
Extracellular polymeric substances
- GP:
-
Generalized polarization
- HaCaT:
-
Human epidermal keratinocyte line
- MRSA:
-
Methicillin-resistant Staphylococcus aureus
- MβCD:
-
Methyl-β-cyclodextrine
- PCL-mPEG:
-
Poly(ε-caprolactone)-monomethoxyl poly (ethylene glycol)
- QR:
-
Combination of quercetin and rifampicin
- QRMs:
-
Quercetin and rifampicin loaded micelles
- Que:
-
Quercetin
- Rif:
-
Rifampicin
- ROS:
-
Reactive oxygen species
- TEM:
-
Transmission electron microscope
References
O’Toole G, Kaplan HB, Kolter R. Biofilm formation as microbial development. Annual Reviews in Microbiology. 2000;54(1):49–79.
Fridman O, Goldberg A, Ronin I, Shoresh N, Balaban NQ. Optimization of lag time underlies antibiotic tolerance in evolved bacterial populations. Nature. 2014;513(7518):418–21.
Morgan DJ, Okeke IN, Laxminarayan R, Perencevich EN, Weisenberg S. Non-prescription antimicrobial use worldwide: a systematic review. Lancet Infect Dis. 2011;11(9):692–701.
Organization WH. Antibiotic resistance: Multi-country public awareness survey. 2015.
Qing G, Zhao X, Gong N, Chen J, Li X, Gan Y, Wang Y, Zhang Z, Zhang Y, Guo W. Thermo-responsive triple-function nanotransporter for efficient chemo-photothermal therapy of multidrug-resistant bacterial infection. Nat Commun. 2019;10(1):1–12.
Dryden M, Baguneid M, Eckmann C, Corman S, Stephens J, Solem C, Li J, Charbonneau C, Baillon-Plot N, Haider S. Pathophysiology and burden of infection in patients with diabetes mellitus and peripheral vascular disease: focus on skin and soft-tissue infections. Clin Microbiol Infect. 2015;21:S27–32.
Kalligeros M, Shehadeh F, Karageorgos SA, Zacharioudakis IM, Mylonakis E. MRSA colonization and acquisition in the burn unit: A systematic review and meta-analysis. Burns. 2019;45(7):1528–36.
Turner NA, Sharma-Kuinkel BK, Maskarinec SA, Eichenberger EM, Shah PP, Carugati M, Holland TL, Fowler VG. Methicillin-resistant Staphylococcus aureus: an overview of basic and clinical research. Nat Rev Microbiol. 2019;17(4):203–18.
Chen M, Xie S, Wei J, Song X, Ding Z, Li X. Antibacterial micelles with vancomycin-mediated targeting and pH/lipase-triggered release of antibiotics. ACS Appl Mater Interfaces. 2018;10(43):36814–23.
Xi Y, Song T, Tang S, Wang N, Du J. Preparation and antibacterial mechanism insight of polypeptide-based micelles with excellent antibacterial activities. Biomacromol. 2016;17(12):3922–30.
Albright V, Xu M, Palanisamy A, Cheng J, Stack M, Zhang B, Jayaraman A, Sukhishvili SA, Wang H. Micelle-coated, hierarchically structured nanofibers with dual-release capability for accelerated wound healing and infection control. Adv Healthcare Mater. 2018;7(11):1800132.
Hu C, Zhang F, Long L, Kong Q, Luo R, Wang Y. Dual-responsive injectable hydrogels encapsulating drug-loaded micelles for on-demand antimicrobial activity and accelerated wound healing. J Control Release. 2020;324:204–17.
Yokoyama M. Polymeric micelles as drug carriers: their lights and shadows. J Drug Target. 2014;22(7):576–83.
Peulen T-O, Wilkinson KJ. Diffusion of nanoparticles in a biofilm. Environ Sci Technol. 2011;45(8):3367–73.
Li J, Zhang K, Ruan L, Chin SF, Wickramasinghe N, Liu H, Ravikumar V, Ren J, Duan H, Yang L. Block copolymer nanoparticles remove biofilms of drug-resistant gram-positive bacteria by nanoscale bacterial debridement. Nano Lett. 2018;18(7):4180–7.
Tian S, Su L, Liu Y, Cao J, Yang G, Ren Y, Huang F, Liu J, An Y, van der Mei HC. Self-targeting, zwitterionic micellar dispersants enhance antibiotic killing of infectious biofilms—An intravital imaging study in mice. Science advances. 2020;6(33):eabb1112.
Liu Y, Busscher HJ, Zhao B, Li Y, Zhang Z, van der Mei HC, Ren Y, Shi L. Surface-adaptive, antimicrobially loaded, micellar nanocarriers with enhanced penetration and killing efficiency in staphylococcal biofilms. ACS Nano. 2016;10(4):4779–89.
Guo P, Xue HY, Buttaro BA, Tran NT, Wong HL. Enhanced eradication of intracellular and biofilm-residing methicillin-resistant Staphylococcus aureus (MRSA) reservoirs with hybrid nanoparticles delivering rifampicin. International Journal of Pharmaceutics. 2020;589:119784.
Dunne WM Jr, Mason EO Jr, Kaplan SL. Diffusion of rifampin and vancomycin through a Staphylococcus epidermidis biofilm. Antimicrob Agents Chemother. 1993;37(12):2522–6.
Farooq U, Ahmad T, Khan A, Sarwar R, Shafiq J, Raza Y, Ahmed A, Ullah S, Rehman NU, Al-Harrasi A. Rifampicin conjugated silver nanoparticles: a new arena for development of antibiofilm potential against methicillin resistant Staphylococcus aureus and Klebsiella pneumoniae. Int J Nanomed. 2019;14:3983.
Zimmerli W, Sendi P. Role of rifampin against staphylococcal biofilm infections in vitro, in animal models, and in orthopedic-device-related infections. Antimicrob Agents Chemother. 2019;63(2):e01746-e1718.
Forrest GN, Tamura K. Rifampin combination therapy for nonmycobacterial infections. Clin Microbiol Rev. 2010;23(1):14–34.
Polerà N, Badolato M, Perri F, Carullo G, Aiello F. Quercetin and its natural sources in wound healing management. Curr Med Chem. 2019;26(31):5825–48.
Song J-Y, Yang B-S. Quercetin shows the pharmacological activity to simultaneously downregulate the inflammatory and fibrotic responses to tissue injury in association with its ability to target multi-kinases. Pharmacology. 2018;102:142–53.
Yin G, Wang Z, Wang Z, Wang X. Topical application of quercetin improves wound healing in pressure ulcer lesions. Exp Dermatol. 2018;27(7):779–86.
Ahmed OM, Mohamed T, Moustafa H, Hamdy H, Ahmed RR, Aboud E. Quercetin and low level laser therapy promote wound healing process in diabetic rats via structural reorganization and modulatory effects on inflammation and oxidative stress. Biomed Pharmacother. 2018;101:58–73.
Andres S, Pevny S, Ziegenhagen R, Bakhiya N, Schäfer B, Hirsch-Ernst KI, Lampen A. Safety aspects of the use of quercetin as a dietary supplement. Mol Nutr Food Res. 2018;62(1):1700447.
Verma A, Stellacci F. Effect of surface properties on nanoparticle–cell interactions. small. 2010;6(1):12–21.
Cui C, Xue Y-N, Wu M, Zhang Y, Yu P, Liu L, Zhuo R-X, Huang S-W. Cellular uptake, intracellular trafficking, and antitumor efficacy of doxorubicin-loaded reduction-sensitive micelles. Biomaterials. 2013;34(15):3858–69.
Zhang Z, Xiong X, Wan J, Xiao L, Gan L, Feng Y, Xu H, Yang X. Cellular uptake and intracellular trafficking of PEG-b-PLA polymeric micelles. Biomaterials. 2012;33(29):7233–40.
Harush-Frenkel O, Debotton N, Benita S, Altschuler Y. Targeting of nanoparticles to the clathrin-mediated endocytic pathway. Biochem Biophys Res Commun. 2007;353(1):26–32.
Gao W, Vecchio D, Li J, Zhu J, Zhang Q, Fu V, Li J, Thamphiwatana S, Lu D, Zhang L. Hydrogel containing nanoparticle-stabilized liposomes for topical antimicrobial delivery. ACS Nano. 2014;8(3):2900–7.
Schmid-Wendtner M-H, Korting HC. The pH of the skin surface and its impact on the barrier function. Skin pharmacology and physiology. 2006;19(6):296–302.
Ang LF, Yam MF, Fung YTT, Kiang PK, Darwin Y. HPLC method for simultaneous quantitative detection of quercetin and curcuminoids in traditional chinese medicines. Journal of pharmacopuncture. 2014;17(4):36.
Goutal S, Auvity S, Legrand T, Hauquier F, Cisternino S, Chapy H, Saba W, Tournier N. Validation of a simple HPLC-UV method for rifampicin determination in plasma: Application to the study of rifampicin arteriovenous concentration gradient. J Pharm Biomed Anal. 2016;123:173–8.
Xiong M-H, Bao Y, Yang X-Z, Zhu Y-H, Wang J. Delivery of antibiotics with polymeric particles. Adv Drug Deliv Rev. 2014;78:63–76.
Patel J, Weinstein M, Eliopoulos G, Jenkins S, Lewis J, Limbago B. M100 Performance standards for antimicrobial susceptibility testing. United State: Clinical and Laboratory Standards Institute. 2017;240.
Hamamoto H, Urai M, Ishii K, Yasukawa J, Paudel A, Murai M, Kaji T, Kuranaga T, Hamase K, Katsu T. Lysocin E is a new antibiotic that targets menaquinone in the bacterial membrane. Nat Chem Biol. 2015;11(2):127–33.
Liu J, Chen Z, Wang J, Li R, Li T, Chang M, Yan F, Wang Y. Encapsulation of curcumin nanoparticles with MMP9-responsive and thermos-sensitive hydrogel improves diabetic wound healing. ACS Appl Mater Interfaces. 2018;10(19):16315–26.
Wei T, Chen C, Liu J, Liu C, Posocco P, Liu X, Cheng Q, Huo S, Liang Z, Fermeglia M. Anticancer drug nanomicelles formed by self-assembling amphiphilic dendrimer to combat cancer drug resistance. Proc Natl Acad Sci. 2015;112(10):2978–83.
Angiolini L, Agnes M, Cohen B, Yannakopoulou K, Douhal A. Formation, characterization and pH dependence of rifampicin: heptakis (2, 6-di-O-methyl)-β-cyclodextrin complexes. Int J Pharm. 2017;531(2):668–75.
Khan MF, Rita SA, Kayser M, Islam M, Asad S, Bin Rashid R, Bari M, Rahman MM, Aman A, Anwar D. Theoretically guided analytical method development and validation for the estimation of rifampicin in a mixture of isoniazid and pyrazinamide by UV spectrophotometer. Front Chem. 2017;5:27.
Sengupta B, Sengupta PK. The interaction of quercetin with human serum albumin: a fluorescence spectroscopic study. Biochem Biophys Res Commun. 2002;299(3):400–3.
Mehranfar F, Bordbar A-K, Parastar H. A combined spectroscopic, molecular docking and molecular dynamic simulation study on the interaction of quercetin with β-casein nanoparticles. J Photochem Photobiol, B. 2013;127:100–7.
Abee T, Kovács ÁT, Kuipers OP, Van der Veen S. Biofilm formation and dispersal in Gram-positive bacteria. Curr Opin Biotechnol. 2011;22(2):172–9.
Min J, Choi KY, Dreaden EC, Padera RF, Braatz RD, Spector M, Hammond PT. Designer dual therapy nanolayered implant coatings eradicate biofilms and accelerate bone tissue repair. ACS Nano. 2016;10(4):4441–50.
Xiao J, Klein MI, Falsetta ML, Lu B, Delahunty CM, Yates III JR, Heydorn A, Koo H. The exopolysaccharide matrix modulates the interaction between 3D architecture and virulence of a mixed-species oral biofilm. PLoS pathogens. 2012;8(4):e1002623.
Shafahi M, Vafai K. Biofilm affected characteristics of porous structures. Int J Heat Mass Transf. 2009;52(3–4):574–81.
Li Y, Liu G, Wang X, Hu J, Liu S. Enzyme-responsive polymeric vesicles for bacterial-strain-selective delivery of antimicrobial agents. Angew Chem Int Ed. 2016;55(5):1760–4.
Liu Y, Ding S, Dietrich R, Märtlbauer E, Zhu K. A biosurfactant-inspired heptapeptide with improved specificity to kill MRSA. Angew Chem. 2017;129(6):1508–12.
Oh G-S, Kim H-J, Choi J-H, Shen A, Choe S-K, Karna A, Lee SH, Jo H-J, Yang S-H, Kwak TH. Pharmacological activation of NQO1 increases NAD+ levels and attenuates cisplatin-mediated acute kidney injury in mice. Kidney Int. 2014;85(3):547–60.
Farha MA, Verschoor CP, Bowdish D, Brown ED. Collapsing the proton motive force to identify synergistic combinations against Staphylococcus aureus. Chem Biol. 2013;20(9):1168–78.
Sanchez SA, Tricerri MA, Gratton E. Laurdan generalized polarization fluctuations measures membrane packing micro-heterogeneity in vivo. Proc Natl Acad Sci. 2012;109(19):7314–9.
Strahl H, Errington J. Bacterial membranes: structure, domains, and function. Annu Rev Microbiol. 2017;71:519–38.
Jeffet U, Shimon R, Sterer N. Effect of high intensity blue light on fusobacterium nucleatum membrane integrity. Photochem Photobiol. 2020;96(1):178–81.
Imlay JA. Pathways of oxidative damage. Annual Reviews in Microbiology. 2003;57(1):395–418.
Hlaing SP, Kim J, Lee J, Hasan N, Cao J, Naeem M, Lee EH, Shin JH, Jung Y, Lee B-L. S-Nitrosoglutathione loaded poly (lactic-co-glycolic acid) microparticles for prolonged nitric oxide release and enhanced healing of methicillin-resistant Staphylococcus aureus-infected wounds. Eur J Pharm Biopharm. 2018;132:94–102.
Hasan N, Cao J, Lee J, Hlaing SP, Oshi MA, Naeem M, Ki M-H, Lee BL, Jung Y, Yoo J-W. Bacteria-targeted clindamycin loaded polymeric nanoparticles: effect of surface charge on nanoparticle adhesion to MRSA, antibacterial activity, and wound healing. Pharmaceutics. 2019;11(5):236.
Sun BK, Siprashvili Z, Khavari PA. Advances in skin grafting and treatment of cutaneous wounds. Science. 2014;346(6212):941–5.
Wang Z, Zhang G, Le Y, Ju J, Zhang P, Wan D, Zhao Q, Jin G, Su H, Liu J. Quercetin promotes human epidermal stem cell proliferation through the estrogen receptor/β-catenin/c-Myc/cyclin A2 signaling pathway. Acta Biochim Biophys Sin. 2020;52(10):1102–10.
Hillaireau H, Couvreur P. Nanocarriers’ entry into the cell: relevance to drug delivery. Cell Mol Life Sci. 2009;66(17):2873–96.
He Z, Liu K, Manaloto E, Casey A, Cribaro GP, Byrne HJ, Tian F, Barcia C, Conway GE, Cullen PJ. Cold atmospheric plasma induces ATP-dependent endocytosis of nanoparticles and synergistic U373MG cancer cell death. Sci Rep. 2018;8(1):1–11.
Bouley R, Yui N, Terlouw A, Cheung PW, Brown D. Chlorpromazine Induces Basolateral Aquaporin-2 Accumulation via F-Actin Depolymerization and Blockade of Endocytosis in Renal Epithelial Cells. Cells. 2020;9(4):1057.
Macia E, Ehrlich M, Massol R, Boucrot E, Brunner C, Kirchhausen T. Dynasore, a cell-permeable inhibitor of dynamin. Dev Cell. 2006;10(6):839–50.
Pelkmans L. Secrets of caveolae-and lipid raft-mediated endocytosis revealed by mammalian viruses. Biochimica et Biophysica Acta (BBA)-Molecular Cell Research. 2005;1746(3):295–304.
Zhang F, Guo H, Zhang J, Chen Q, Fang Q. Identification of the caveolae/raft-mediated endocytosis as the primary entry pathway for aquareovirus. Virology. 2018;513:195–207.
Lin H, Wang Q, Zhong R, Li Z, Zhao W, Chen Y, Tian M, Luo X. Biomimetic phosphorylcholine strategy to improve the hemocompatibility of pH-responsive micelles containing tertiary amino groups. Colloids and Surfaces B: Biointerfaces. 2019;184:110545.
Yu T, Malugin A, Ghandehari H. Impact of silica nanoparticle design on cellular toxicity and hemolytic activity. ACS Nano. 2011;5(7):5717–28.
Zhen Z, Liu X, Huang T, Xi T, Zheng Y. Hemolysis and cytotoxicity mechanisms of biodegradable magnesium and its alloys. Mater Sci Eng, C. 2015;46:202–6.
Baskurt OK, Meiselman HJ. Erythrocyte aggregation: basic aspects and clinical importance. Clin Hemorheol Microcirc. 2013;53(1–2):23–37.
Tian Y, Tian Z, Dong Y, Wang X, Zhan L. Current advances in nanomaterials affecting morphology, structure, and function of erythrocytes. RSC Adv. 2021;11(12):6958–71.
Acknowledgements
The authors have no conflicts of interest to declare that are relevant to the content of this article. The laboratory animals (mice) used in our experiments were in accordance with the relevant Chinese laws and according to the China Agriculture University regulations concerning protection of animals used for scientific purposes (2010-SYXK-0037). The mice used in this study were approved by the Ethics Committee on Experimental Animals and Animal Tests of China Agricultural University. The review number is AW22011202-2-1.
Funding
This research was supported by the National Key Research and Development Program of China (No: 2021YFD1801000). Chinese National Natural Science Foundation project (No: 31971312 and No: 32171389), China Agriculture Research System of MOF and MARA (CARS-36), and the 2115 Talent Development Program of China Agricultural University.
Author information
Authors and Affiliations
Corresponding authors
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
About this article
Cite this article
Chen, Y., Zhao, Q., Han, J. et al. Dual Drug Loaded pH-sensitive Micelles for Efficient Bacterial Infection Treatment. Pharm Res 39, 1165–1180 (2022). https://doi.org/10.1007/s11095-022-03182-5
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11095-022-03182-5