Abstract
Purpose
There is ongoing concern regarding increased toxicity from paclitaxel in elderly patients, particularly of severe neutropenia. Yet, data so far is controversial and this concern is not supported by a clinically relevant age-dependent difference in pharmacokinetics (PK) of paclitaxel. This study assessed whether age is associated with increased risk for paclitaxel-induced neutropenia.
Methods
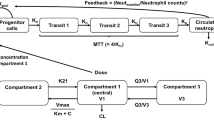
Paclitaxel plasma concentration-time data, pooled from multiple different studies, was combined with available respective neutrophil count data during the first treatment cycle. Paclitaxel pharmacokinetic-pharmacodynamic (PK-PD) data was modeled using a non-linear mixed effects approach and a semiphysiological neutropenia model, where systemic paclitaxel exposure was linked to reduced proliferation of neutrophils. The impact of age was evaluated on relevant variables in the model, using a significance threshold of p < 0.005.
Results
Paclitaxel PK-PD data was evaluated from 300 patients, with a median age of 65 years (range 23–84 years), containing 116 patients ≥70 years (39%). First cycle neutrophil counts were adequately described by a threshold effect model of paclitaxel on the proliferation rate of neutrophils. Age as a continuous or dichotomous variable (≥70 versus <70 years) did not significantly impact sensitivity of the bone marrow to paclitaxel nor the average maturation time of neutrophils (both p > 0.005), causing a decline in the respective interindividual variability of <1%.
Conclusion
Results from this large retrospective patient cohort do not suggest elderly patients to be at an increased risk of developing paclitaxel-associated neutropenia during the first treatment cycle. Reflexive dose reductions of paclitaxel in elderly patients are unlikely to improve the risk of severe neutropenia and may be deleterious.




Similar content being viewed by others
Abbreviations
- ANCbase :
-
Baseline absolute neutrophil count
- ANCt :
-
Absolute neutrophil count at time t
- Cdrug :
-
Systemic drug concentration
- EC50 :
-
Drug concentration at half the maximum inhibitory effect
- E drug :
-
Drug effect
- Emax :
-
Maximum inhibiting drug effect
- Erasmus MC:
-
Erasmus Medical Center Cancer Institute
- FB:
-
Feedback
- IIV:
-
Interindividual variability
- k prol :
-
Rate constant for the proliferation of neutrophils
- k tr :
-
Rate constant for the maturation of neutrophils
- MTT:
-
Mean transit time
- NKI:
-
Netherlands Cancer Institute
- PK-PD:
-
Pharmacokinetic-pharmacodynamic
- Radboud UMC :
-
Radboud University Medical Center
- SLOPE:
-
Slope factor of linear drug effect
- Tc > 0.05μM :
-
Time-above-threshold concentration of 0.05 μmol/L
References
Food and Drug Administration. TAXOL (paclitaxel), NDA 020262. 2011.
Lichtman SM, Hollis D, Miller A A, Rosner GL, Rhoades C A, Lester EP, et al. Prospective evaluation of the relationship of patient age and paclitaxel clinical pharmacology: Cancer and leukemia group B (CALGB 9762). J Clin Oncol 2006;24:1846–1851.
Lichtman SM, Hurria A, Cirrincione CT, Seidman AD, Winer E, Hudis C, et al. Paclitaxel efficacy and toxicity in older women with metastatic breast cancer: combined analysis of calgb 9342 and 9840. Ann Oncol. 2012;23:632–8.
Nakamura Y, Sekine I, Furuse K, Saijo N. Retrospective comparison of toxicity and efficacy in phase II trials of 3-h infusions of paclitaxel for patients 70 years of age or older and patients under 70 years of age. Cancer Chemother Pharmacol. 2000;46:114–8.
Fidias P, Supko JG, Martins R, Boral A, Carey R, Grossbard M, et al. Measurement and impact of co-morbidity in elderly patients with advanced non-small cell lung cancer treated with chemotherapy. A phase II study of weekly paclitaxel. Clin Cancer Res. 2001;7:3942–9.
Smorenburg CH, Ten Tije AJ, Verweij J, Bontenbal M, Mross K, Van Zomeren DM, et al. Altered clearance of unbound paclitaxel in elderly patients with metastatic breast cancer. Eur J Cancer. 2003;39:196–202.
Nieuweboer AJ, Smid M, de Graan A-JM, Elbouazzaoui S, de Bruijn P, Martens JW, et al. Predicting paclitaxel-induced neutropenia using the DMET platform. Pharmacogenomics. 2015;16:1231–41.
Sehl M, Sawhney R, Naeim A. Physiologic aspects of aging: impact on cancer management and decision making, part II. Cancer J. 2005;11:461–73.
Aapro M, Skacel T, von Minckwitz G, Lawrinson S, Schwenkglenks M, Lyman GH, et al. Pegfilgrastim primary prophylaxis vs. current practice neutropenia management in elderly breast cancer patients receiving chemotherapy. Crit Rev Oncol Hematol. 2009;74:203–10.
Crombag M-RB, de Vries SA, Koolen SL, Wijngaard S, Joerger M, Schellens JH, et al. Impact of older age on the exposure of paclitaxel: a population pharmacokinetic study. Pharm Res. 2019;36.
Gianni BL, Kearns CM, Giani A, Capri G, Vigan L, Locatelli A, et al. Nonlinear pharmacokinetics and metabolism of paclitaxel and its pharmacokinetic/Pharmacodynamic relationships in humans. J Clin Oncol. 1995;13:180–90.
Ohtsu T, Sasaki Y, Tamura T, Nishiwaki Y, Saijo N. Clinical pharmacokinetics and pharmacodynamics of paclitaxel: A 3-hour infusion versus a 24-hour infusion. Clin Cancer Res. 1995;1:599–606.
Xin D, Zhou L, Li C, Zhang S, Huang H, Qiu G, et al. TC > 0.05 as a pharmacokinetic parameter of paclitaxel for therapeutic efficacy and toxicity in Cancer patients. Recent Pat Anticancer Drug Discov. 2018;13:341–7.
Joerger M, Kraff S, Huitema ADR, Feiss G, Moritz B, Schellens JHM, et al. Evaluation of a pharmacology-driven dosing algorithm of 3-weekly paclitaxel using therapeutic drug monitoring: A pharmacokinetic-pharmacodynamic simulation study. Clin Pharmacokinet. 2012;51:607–17.
De Graan AJM, Elens L, Smid M, Martens JW, Sparreboom A, Nieuweboer AJM, et al. A pharmacogenetic predictive model for paclitaxel clearance based on the DMET platform. Clin Cancer Res. 2013;19:5210–7.
De Graan AJM, Elens L, Sprowl JA, Sparreboom A, Friberg LE, Van Der Holt B, et al. CYP3A4*22 genotype and systemic exposure affect paclitaxel-induced neurotoxicity. Clin Cancer Res. 2013;19:3316–24.
Joerger M, Huitema ADR, van den Bongard DHJG, Schellens JHM, Beijnen JH. Quantitative effect of gender, age, liver function, and body size on the population pharmacokinetics of paclitaxel in patients with solid tumors. Clin Cancer Res. 2006;12:2150–7.
Kraff S, Nieuweboer AJM, Mathijssen RHJ, Baty F, De Graan AJ, Van Schaik RHN, et al. Pharmacokinetically based dosing of weekly paclitaxel to reduce drug-related neurotoxicity based on a single sample strategy. Cancer Chemother Pharmacol. 2015;75:975–83.
The International Conference on Harmonisation of Technical Requirements for Registration of Pharmaceuticals for Human Use. (ICH) 2018. www.ich.org.
Friberg LE, Henningsson A, Maas H, Nguyen L, Karlsson MO. Model of chemotherapy-induced myelosuppression with parameter consistency across drugs. J Clin Oncol. 2002;20:4713–21.
Henrich A, Joerger M, Kraff S, Jaehde U, Huisinga W, Kloft C, et al. Semimechanistic bone marrow exhaustion pharmacokinetic/Pharmacodynamic model for chemotherapy-induced cumulative neutropenia s. J Pharmacol Exp Ther J Pharmacol Exp Ther. 2017;362:347–58.
Mielke S, Sparreboom A, Steinberg SM, Gelderblom H, Unger C, Behringer D, et al. Association of Paclitaxel Pharmacokinetics with the development of peripheral neuropathy in patients with advanced Cancer. Clin Cancer Res. 2005;11:4843–50.
Mielke S, Mross K, Gerds T A, Schmidt A, Wäsch R, Berger DP, et al. Comparative neurotoxicity of weekly non-break paclitaxel infusions over 1 versus 3 h. Anti-Cancer Drugs 2003;14:785–792.
Joerger M, Von Pawel J, Kraff S, Fischer JR, Eberhardt W, Gauler TC. Open-label, randomised study of individualized, pharmacokinetically (PK)-guided dosing of paclitaxel combined with carboplatin or cisplatin in patients with advanced non-small cell lung cancer (NSCLC). Ann Oncol. 2016;27:1895–902.
Mielke S, Sparreboom A, Behringer D, Mross K. Paclitaxel pharmacokinetics and response to chemotherapy in patients with advanced cancer treated with a weekly regimen. Anticancer Res. 2005;25:4423–7.
Joerger M, Huitema ADR, Richel DJ, Dittrich C, Pavlidis N, Briasoulis E, et al. Population pharmacokinetics and pharmacodynamics of paclitaxel and carboplatin in ovarian cancer patients: A study by the European organization for research and treatment of cancer-pharmacology and molecular mechanisms group and new drug development group. Clin Cancer Res. 2007;13:6410–8.
Dansirikul C, Silber HE, Karlsson MO. Approaches to handling pharmacodynamic baseline responses. J Pharmacokinet Pharmacodyn. 2008;35:269–83. https://doi.org/10.1007/s10928-008-9088-2.
Dosne AG, Bergstrand M, Harling K, Karlsson MO. Improving the estimation of parameter uncertainty distributions in nonlinear mixed effects models using sampling importance resampling. J Pharmacokinet Pharmacodyn. 2016;43:583–96.
de Graan A-JM, Loos WJ, Friberg LE, Baker SD, van der Bol JM, van Doorn L, et al. Influence of smoking on the pharmacokinetics and toxicity profiles of Taxane therapy. Clin Cancer Res. 2012;18:4425–32.
Fontanella C, Bolzonello S, Lederer B, Aprile G. Management of breast cancer patients with chemotherapy-induced neutropenia or febrile neutropenia. Breast Care. 2014;9:239–45.
Pettengell R, Schwenkglenks M, Leonard R, Bosly A, Paridaens R, Constenla M, et al. Neutropenia occurrence and predictors of reduced chemotherapy delivery: results from the INC-EU prospective observational European neutropenia study. Support Care Cancer. 2008;16:1299–309.
Rivera E, Haim Erder M, Fridman M, Frye D, Hortobagyi GN. First-cycle absolute neutrophil count can be used to improve chemotherapy-dose delivery and reduce the risk of febrile neutropenia in patients receiving adjuvant therapy: a validation study. Breast Cancer Res. 2003;5:114–20. https://doi.org/10.1186/bcr618.
Belani BCP, Kearns CM, Zuhowski EG, Erkmen K, Hiponia D, Zacharski D, et al. Phase I Trial , Including Pharmacokinetic and Pharmacodynamic Correlations, of Combination Paclitaxel and Carboplatin in Patients With Metastatic Non–Small-Cell Lung Cancer. J Clin Oncol 1999;17:676–684
Huizing MT, van Warmerdam LJ, Rosing H, Schaefers MC, Lai A, Helmerhorst TJ, et al. Phase I and pharmacologic study of the combination paclitaxel and carboplatin as first-line chemotherapy in stage III and IV ovarian cancer. J Clin Oncol. 1997;15:1953–64.
Hurria A, Mohile S, Gajra A, Klepin H, Muss H, Chapman A, et al. Validation of a prediction tool for chemotherapy toxicity in older adults with Cancer. J Clin Oncol. 2016;34:2366–71.
ACKNOWLEDGMENTS AND DISCLOSURES
The authors wish to express their gratitude to all study participants and health care employees involved in patient inclusion, with special thanks to Ellen van der Pan and Anoek van Straten. The authors thank the Research High Performance Computing facility of the NKI for support in the use of computational resources. Jos H. Beijnen is a (part-time) employee and shareholder of Modra Pharmaceuticals, and (partly) holds a patent on oral taxane pharmaceutical formulations. The other authors declare no conflicts of interest in connection with this manuscript. All procedures performed in studies involving human participants were in accordance with the ethical standards of the institutional research committees and was carried out in accordance with ICH Guidelines for Good Clinical Practice. Written informed consent was obtained from all individual participants.
Author information
Authors and Affiliations
Corresponding author
Additional information
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
About this article
Cite this article
Crombag, MR.B.S., Koolen, S.L.W., Wijngaard, S. et al. Does Older Age Lead to Higher Risk for Neutropenia in Patients Treated with Paclitaxel?. Pharm Res 36, 163 (2019). https://doi.org/10.1007/s11095-019-2697-1
Received:
Accepted:
Published:
DOI: https://doi.org/10.1007/s11095-019-2697-1