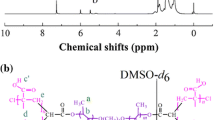
ABSTRACT
Purpose
Block copolymer micelles are extensively used as drug controlled release carriers, showing promising application prospects. The comb or brush copolymers are especially of great interest, whose densely-grafted side chains may be important for tuning the physicochemical properties and conformation in selective solvents, even in vitro drug release. The purpose of this work was to synthesize novel block copolymer combs via atom transfer radical polymerization, to evaluate its physicochemical features in solution, to improve drug release behavior and to enhance the bioavailablity, and to decrease cytotoxicity.
Methods
The physicochemical properties of the copolymer micelles were examined by modulating the composition and the molecular weights of the building blocks. A dialysis method was used to load hydrophobic camptothecin (CPT), and the CPT release and stability were detected by UV–vis spectroscopy and high-performance liquid chromatography, and the cytotoxicity was evaluated by MTT assays.
Results
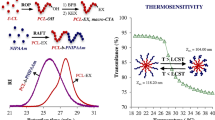
The copolymers could self-assemble into well-defined spherical core-shell micelle aggregates in aqueous solution, and showed thermo-induced micellization behavior, and the critical micelle concentration was 2.96–27.64 mg L−1. The micelles were narrow-size-distribution, with hydrodynamic diameters about 128–193 nm, depending on the chain length of methoxy polyethylene glycol (mPEG) blocks and poly(N-isopropylacrylamide) (PNIPAM) graft chains or/and compositional ratios of mPEG to PNIPAM. The copolymer micelles could stably and effectively load CPT but avoid toxicity and side-effects, and exhibited thermo-dependent controlled and targeted drug release behavior.
Conclusions
The copolymer micelles were safe, stable and effective, and could potentially be employed as CPT controlled release carriers.








Similar content being viewed by others
References
Sanada Y, Akiba I, Sakurai K, Shiraishi K, Yokoyama M, Mylonas E, et al. Hydrophobic molecules infiltrating into the poly(ethylene glycol) domain of the core/shell interface of a polymeric micelle: evidence obtained with anomalous small-angle X-ray scattering. J Am Chem Soc. 2013;135(7):2574–82.
Wang C, Long C, Xie C, Chen X, Zhang L, Chu B, et al. Two novel nanoscale preparations of micelle and thermosensitive hydrogel for docetaxel to treat malignant tumor. J Biomed Nanotechnol. 2013;9(3):357–66.
Peng J, Qi T, Liao J, Fan M, Luo F, Li H, et al. Synthesis and characterization of novel dual-responsive nanogels and their application as drug delivery systems. Nanoscale. 2012;4(8):2694–704.
Kawano K, Watanabe M, Yamamoto T, Yokoyama M, Opanasopit P, Okano T, et al. Enhanced antitumor effect of camptothecin loaded in long-circulating polymeric micelles. J Control Rel. 2006;112:329–32.
Kim DJ, Heo JY, Kim KS, Choi IS. Formation of thermoresponsive poly (N-iso propylacrylamide)/dextran particles by atom transferradical polymerization. Macromol Rapid Commun. 2003;24:517–21.
Gil ES, Hudson SM. Stimuli-responsive polymers and their bioconjugates. Prog Polym Sci. 2004;29:1173–222.
Alarcon CH, Pennadam S, Alexander C. Stimuli responsive polymers for biomedical applications. Chem Soc Rev. 2005;34:276–85.
Rezaei SJT, Nabid MR, Niknejad H, Entezami AA. Multifunctional and thermo-responsive unimolecular micelles for tumor-targeted delivery and site-specifically release of anticancer drugs. Polymer. 2012;53:3485–97.
Opanasopit P, Yokoyama M, Watanabe W, Kawano K, Maitani Y, Okano T. Influence of serum and albumins from different species on stability of camptothecin-loaded micelles. J Control Release. 2005;104:313–21.
Kim S, Shi Y, Kim JY, Park K, Cheng JX. Overcoming the barriers in micellar drug delivery: loading efficiency, in vivo stability, and micelle-cell interaction. Expert Opin Drug Del. 2010;7:49–62.
Rapoport N. Physical stimuli-respons ive polymeric micelles for anti-cancer drug delivery. Prog Polym Sci. 2007;32:962–90.
Attia ABE, Ong ZY, Hedrick JL, Lee PP, Ee PLR, Hammond PT, et al. Mixed micelles self-assembled from block copolymers for drug delivery. Cur Opin Colloid Interface Sci. 2011;16:182–94.
Konovalov MA, Kramarenko EY, Khokhlov AR, Reineker P. Conformational behavior of diblock comb copolymers. J Chem Phys. 2009;130:164903–9.
Zhao J, Mountrichas G, Zhang G, Pispas S. Thermoresponsive core–shell brush copolymers with poly(propylene oxide)-block-poly(ethylene oxide) side chains via a ‘grafting from’ technique. Macromolecules. 2010;43(4):1771–7.
Li X, Kong X, Zhang J, Wang Y, Wang Y, Shi S, et al. A novel composite hydrogel based on chitosan and inorganic phosphate for local drug delivery of camptothecin nanocolloids. J Pharm Sci. 2011;100(1):232–41.
Tang DL, Song F, Chen C, Wang XL, Wang YZ. A pH-responsive chitosan-b-poly(p-dioxanone) nanocarrier: formation and efficient antitumor drug delivery. Nanotechnology. 2013;24(14):145101–10.
Lu J, Liong M, Li Z, Zink JI, Tamanoi F. Biocompatibility, biodistribution, and drug-delivery efficiency of mesoporous silica nanoparticles for cancer therapy in animals. Small. 2010;6(16):1794–805.
Luo YL, Wei Y, Xu F, Zhang LL. Novel thermo-responsive self-assembly micelles from a double brush-shaped PNIPAM-g-(PA-b-PEG-b-PA)-g-PNIPAM copolymer via an atom transfer radical polymerization route. J Polym Sci, Part A: Polym Chem. 2012;50(10):2053–67.
Wei H, Zhang XZ, Cheng C, Cheng SX, Zhuo RX. Self-assembled, thermosensitive micelles of a star block copolymer based on PMMA and PNIPAAm for controlled drug delivery. Biomaterials. 2007;28:99–107.
Venkataraman S, Hedrick JL, Ong ZY, Yang C, Ee PLR, Hammond PT, et al. The effects of polymeric nanostructure shape on drug delivery. Adv Drug Del Rev. 2011;63:1228–46.
Chang YC, Chu IM. Methoxy poly(ethylene glycol)-b-poly(valerolactone) diblock polymeric micelles for enhanced encapsulation and protection of camptothecin. Eur Polym J. 2008;44:3922–30.
Mendez S, Curro JG, McCoy JD, Lopez GP. Computational modeling of the temperature-induced structural changes of tethered poly(N-isopropylacrylamide) with self-consistent field theory. Macromolecules. 2005;38:174–81.
Halperin A. Compression induced phase transitions in PEO brushes: the n-cluster. Model Eur Phys J B. 1998;3:359–64.
Plunkett KN, Zhu X, Moore JS, Leckband DE. PNIPAM Chain collapse depends on the molecular weight and grafting density. Langmuir. 2006;22(9):4259–66.
Dreher MR, Simnick AJ, Fischer K, Smith RJ, Patel A, Schmidt M, et al. Temperature triggered self-assembly of polypeptides into multivalent spherical micelles. J Am Chem Soc. 2008;130(2):687–94.
Cai Q, Zhang L, Yang J, Jin R. Core-crosslinked poly(N-isopropyl -acrylamide-co-N, N-dimethylacrylamide)-b-poly(ε-caprolactone) micelles for paclitaxel thermo-sensitive controlled release behaviors. J Clin Rehabilitative Tissue Eng Res. 2010;14(29):5505–10.
Lee SC, Chang JY. Thermosensitive block copolymers consisting of poly(N-isopropylacrylamide) and star shape oligo(ethylene oxide). Bull Korean Chem Soc. 2009;30(7):1521–5.
Greenwood R, Kendall K. Selection of suitable dispersants for aqueous suspensions of zirconia and titania powders using acoustophoresis. J Eur Ceramic Soc. 1999;19:479–88.
Hanaor D, Michelazzi M, Leonelli C, Sorrell CC. The effects of carboxylic acids on the aqueous dispersion and electrophoretic deposition of ZrO2. J Eur Ceramic Soc. 2012;32:235–324.
Gu JC, Qiao MX, Gao W, Zhao XL, Hu HY, Xu J, et al. Preparation of adriamycin-loaded temperature/pH sensitive self-assembly block copolymer micelles. Acta Pharm Sin. 2009;44:793–7.
Tauer K, Gau D, Schulze S, Völkel A, Dimova R. Thermal property changes of poly(N-isopropylacrylamide) microgel particles and block copolymers. Colloid Polym Sci. 2009;287:299–312.
Luo Y, Li L, Wang Y, Chan G, Hou Z, Zhang Q. Preparation and characteristic of hydroxycamptothecin-loaded PLA nanoparticles using dialysis method. J Xiamen University Nat Sci. 2010;49(6):832–7.
Swaminathan S, Pastero L, Serpe L, Trotta F, Vavia P, Aquilano D, et al. Cyclodextrin-based nanosponges encapsulating camptothecin: physicochemical characterization, stability and cytotoxicity. Eur J Pharm Biopharm. 2010;74:193–201.
Yao R, Liu L, Deng S, Ren W. Preparation of carboxymethylchitosan nanoparticles with acid-sensitive bond based on solid dispersion of 10-hydroxycamptothecin. ISRN Pharm. 2011;2011:1–9. doi:10.5402/2011/624704.
Hevus I, Modgil A, Daniels J, Kohut A, Sun C, Stafslien S, et al. Invertible micellar polymer assemblies for delivery of poorly water-soluble drugs. Biomacromolecules. 2012;13:2537–45.
Min KH, Park K, Kim YS, Bae SM, Lee S, Jo HG, et al. Hydrophobically modified glycol chitosan nanoparticles-encapsulated camptothecin enhance the drug stability and tumor targeting in cancer therapy. J Control Release. 2008;127:208–18.
Jaskari T, Vuorio M, Kontturi K, Manzanares JA, Hirvonen J. Iion-exchange fibers and drugs: an equilibrium study. J Control Release. 2001;70:219–29.
Freiberg S, Zhu XX. Polymer microspheres for controlled drug release. Int J Pharm. 2004;282:1–18.
Opanasopit P, Yokoyama M, Watanabe M, Kawano K, Maitani Y, Okano T. Block copolymer design for camptothecin incorporation into polymeric micelles for passive tumor targeting. Pharm Res. 2004;21:2001–8.
Huang XJ, Xiao Y, Lang MD. Self-assembly of pH-sensitive mixed micelles based on linear and star copolymers for drug delivery. J Colloid Interface Sci. 2011;364:92–9.
Luo YL, Wang SH, Li ZQ. Characterization, microstructure, and gas sensitive response behavior of PEG/lithium salt polymer electrolyte films. J Mater Sci. 2008;43:174–84.
Yahara T, Koga T, Yoshida S, Deguchi T, Shirouzu K. Relationship between microvessel density and thermographic hot areas in breast cancer. Surg Today. 2003;33:243–8.
Akimoto J, Nakayama M, Sakai K, Okano T. Temperature-induced intracellular uptake of thermoresponsive polymeric micelles. Biomacromolecules. 2009;10:1331–6.
Akimoto J, Nakayama M, Sakai K, Okano T. Thermally controlled intracellular uptake system of polymeric micelles possessing poly(N-isopropylacrylamide) -based outer coronas. Mol Pharm. 2010;7:926–35.
Huang P, Zhou J. The enhancement of hypeffhermia study on patient with tumor. Modern Oncology. 2010;18(7):1460–2.
Ritger PL, Peppas NA. A simple equation for description of solute release I. Fickian and non-Fickian release from non-swellable devices in form of slabs, sphere, cylinders or discs. J Control Release. 1987;5:23–36.
Martins SM, Wendling T, Goncalves VMF, Sarmentoa B, Ferreira DC. Development and validation of a simple reversed-phase HPLC method for the determination of camptothecin in animal organs following administration in solid lipid nanoparticles. J Chromatography B. 2012;880:100–7.
Greish K, Fang J, Inutsuka T, Nagamitsu A, Maeda H. Macromolecular therapeutics: advantages and prospects with special emphasis on solid tumour targeting. Clin Pharmacokinet. 2003;42:1089–105.
Liu J, Lee H, Allen C. Formulation of drugs in block copolymer micelles: drug loading and release. Curr Pharm Des. 2006;12(36):4685–701.
Acknowledgments and Disclosures
This work is supported by the Natural Science Foundation of China (grant NSFC 21273142), Natural Science Foundation of Shaanxi Province (2012JM6009), and Graduate Education Innovation Funds (2013CXS049).
Author information
Authors and Affiliations
Corresponding authors
Electronic supplementary material
Below is the link to the electronic supplementary material.
ESM 1
(DOC 86 kb)
Rights and permissions
About this article
Cite this article
Yang, XL., Luo, YL., Xu, F. et al. Thermosensitive mPEG-b-PA-g-PNIPAM Comb Block Copolymer Micelles: Effect of Hydrophilic Chain Length and Camptothecin Release Behavior. Pharm Res 31, 291–304 (2014). https://doi.org/10.1007/s11095-013-1160-y
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11095-013-1160-y